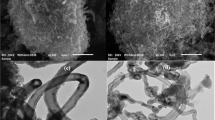
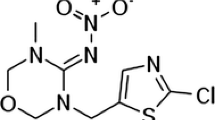
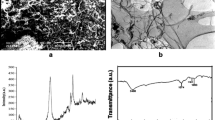
Abstract
The presence of chlorophenols in drinking water can be hazardous to human health. Understanding the mechanisms of adsorption under specific experimental conditions would be beneficial when developing methods to remove toxic substances from drinking water during water treatment in order to limit human exposure to these contaminants. In this study, we investigated the sorption of chlorophenols on multi-walled carbon nanotubes using a density functional theory (DFT) approach. This was applied to study selected interactions between six solvents, five types of nanotubes, and six chlorophenols. Experimental data were used to construct structure–adsorption relationship (SAR) models that describe the recovery process. Specific interactions between solvents and chlorophenols were taken into account in the calculations by using novel specific mixture descriptors.

Similar content being viewed by others
References
Neilson AH, Allard AS, Hynning PA, Remberger M (1991) Distribution, fate, and persitence of organochlorine compounds formed during the production of bleached pulp. Toxicol Environ Chem 30(1-2):3–41. doi:10.1080/02772249109357638
Saitoh T, Kondo T, Hiraide M (2007) Concentration of chlorophenols in water to dialkyated catinonic surfactant–silica gel admicelles. J Chromatogr A 1164(1–2):40–47. doi:10.1016/j.chroma.2007.07.021
Dorathi PJ, Kandasamy P (2012) Dechlorination of chlorophenols by zero valent iron impregnated silica. J Environ Sci 24(4):765–773. doi:10.1016/S1001-0742(11)60817-6
Xing L, Liu H, Giesy JP, Zhang X, Yu H (2012) Probabilistic ecological risk assessment for three chlorophenols in surface waters of China. J Environ Sci 24(2):329–334. doi:10.1016/S1001-0742(11)60779-1
Wang Y-Q, Tan C-Y, Zhuang S-L, Zhai P-Z, Cui Y, Zhou Q-H, Zhang H-M, Fei Z (2014) In vitro and in silico investigations of the binding interactions between chlorophenols and trypsin. J Hazard Mater 278:55–65. doi:10.1016/j.jhazmat.2014.05.092
Selvam NCS, Narayanan S, Kennedy LJ, Vijaya JJ (2013) Pure and mg-doped self-assembled ZnO nanoparticles for the enhanced photocatalytic degradation of 4-chlorophenol. J Environ Sci 25(10):2157–2167. doi:10.1016/S1001-0742(12)60277-0
Zhang X-N, Huang W-M, Wang X, Li H, Lu H-Y, Lin H-B (2011) Biofilm-electrode process with high efficiency for degradation of 2,4-dichlorophenol. Environ Chem Lett 9(3):383–388. doi:10.1007/s10311-010-0290-2
AfTSaD Registry (1999) Public health statement: chlorophenols. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Scow K, Goyer M, Perwak J, Woodruff C, Saterson K, Payne E, Wood M (1982) An exposure and risk assessment for chlorinated phenols: 2-chlorophenol, 2,4-dichlorophenol, 2,4,6-trichlorophenol. Office of Water Regulations and Standards, Washington, DC
Bellar TA, Lichtenberg JJ, Kroner RC (1974) The occurrence of organohalides in chlorinated drinking waters. J Am Water Works Assoc 66(12):703–706. doi:10.2307/41267646
US EPA (2014) Priority pollutant list. United States Environmental Protection Agency, Washington, DC
Ravelo-Pérez LM, Herrera-Herrera AV, Hernández-Borges J, Rodríguez-Delgado MÁ (2010) Carbon nanotubes: solid-phase extraction. J Chromatogr A 1217(16):2618–2641. doi:10.1016/j.chroma.2009.10.083
Liu X, Ji Y, Zhang Y, Zhang H, Liu M (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165(1–2):10–17. doi:10.1016/j.chroma.2007.07.057
Wu Z, Sun C, Chai Y, Zhang M (2011) Fe3O4, SiO2, Pd-Au: a highly efficient and magnetically separable catalyst for liquid-phase hydrodechlorination of 4-chlorophenol. RSC Adv 1(7):1179–1182. doi:10.1039/C1RA00491C
Liu Q, Shi J, Zeng L, Wang T, Cai Y, Jiang G (2011) Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J Chromatogr A 1218(2):197–204. doi:10.1016/j.chroma.2010.11.022
Lacerda L, Bianco A, Prato M, Kostarelos K (2006) Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev 58(14):1460–1470. doi:10.1016/j.addr.2006.09.015
Cantalini C, Valentini L, Armentano I, Lozzi L, Kenny JM, Santucci S (2003) Sensitivity to NO2 and cross-sensitivity analysis to NH3, ethanol and humidity of carbon nanotubes thin film prepared by PECVD. Sensors Actuators B 95:195–202. doi:10.1016/S0925-4005(03)00418-0
Ramraj A, Hillier IH (2010) Binding of pollutant aromatics on carbon nanotubes and graphite. J Chem Inf Model 50(4):585–588. doi:10.1021/ci1000604
Peng X, Li Y, Luan Z, Di Z, Wang H, Tian B, Jia Z (2003) Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem Phys Lett 376:154–158. doi:10.1016/S0009-2614(03)00960-6
Balamurugan K, Subramanian V (2013) Adsorption of chlorobenzene onto (5,5) armchair single-walled carbon nanotube and graphene sheet: toxicity versus adsorption strength. J Phys Chem C 117(41):21217–21227. doi:10.1021/jp403646h
Woods LM, Bădescu ŞC, Reinecke TL (2007) Adsorption of simple benzene derivatives on carbon nanotubes. Phys Rev B 75(15):155415. doi:10.1103/PhysRevB.75.155415
Zou M, Zhang J, Chen J, Li X (2012) Simulating adsorption of organic pollutants on finite (8,0) single-walled carbon nanotubes in water. Environ Sci Technol 46(16):8887–8894. doi:10.1021/es301370f
Sizochenko N, Leszczynski J (2016) Review of current and emerging approaches for quantitative nanostructure–activity relationship modeling: the case of inorganic nanoparticles. J Nanotoxicol Nanomed 1(1):1–16. doi:10.4018/JNN.2016010101
Brasquet C, Le Cloirec P (1999) Effects of activated carbon cloth surface on organic adsorption in aqueous solutions. Use of statistical methods to describe mechanisms. Langmuir 15(18):5906–5912. doi:10.1021/la9811160
Xu L, Ball JW, Dixon SL, Jurs PC (1994) Quantitative structure–activity relationships for toxicity of phenols using regression analysis and computational neural networks. Environ Toxicol Chem 13(5):841–851. doi:10.1002/etc.5620130520
Rofouei MK, Salahinejad M, Ghasemi JB (2014) An alignment independent 3D-QSAR modeling of dispersibility of single-walled carbon nanotubes in different organic solvents. Fuller Nanotub Car N 22(7):605–617. doi:10.1080/1536383X.2012.702157
Salahinejad M, Zolfonoun E (2013) QSAR studies of the dispersion of SWNT in different organic solvents. J Nanopart Res 15(11):1–9. doi:10.1007/s11051-013-2028-0
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160(2–3):265–288. doi:10.1016/j.jhazmat.2008.03.045
Gołębiowski M, Stepnowski P, Leszczynska D (2017) Application of carbon nanotubes as solid phase extraction sorbent for analysis of chlorophenols in water samples. Chem Pap. doi:10.1007/s11696-016-0098-z
Sizochenko N, Majumdar D, Roszak S, Leszczynski J (2016) Application of quantum mechanics and molecular mechanics in chemoinformatics. Handbook of computational chemistry. Springer, Dordrecht. doi:10.1007/978-94-007-6169-8_52-1
Karabulut S, Sizochenko N, Orhan A, Leszczynski J (2016) A DFT-based QSAR study on inhibition of human dihydrofolate reductase. J Molr Graph Model 70:23–29. doi:10.1016/j.jmgm.2016.09.005
Yang K, Xing B (2010) Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem Rev 110(10):5989–6008. doi:10.1021/cr100059s
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford
Tournas F, Charlier JC (2005) Ab initio study of benzene adsorption on carbon nanotubes. Phys Rev B 71(16):165421. doi:10.1103/PhysRevB.71.165421
Coleman WF, Arumainayagam CR (1998) Hyperchem 5 (by Hypercube, Inc.). J Chem Educ 75(4):416. doi:10.1021/ed075p416
Todeschini R, Consonni V, Mauri A, Pavan M (2004) Dragon 5.1. Milano Chemometrics and QSAR Research Group, Milano
Ajmani S, Rogers SC, Barley MH, Livingstone DJ (2006) Application of QSPR to mixtures. J Chem Inf Model 46(5):2043–2055. doi:10.1021/ci050559o
Brüggemann R, Carlsen L (2006) Partial order in environmental sciences and chemistry. Springer, Berlin
Carlsen L (2006) A combined QSAR and partial order ranking approach to risk assessment. SAR QSAR Environ Res 17(2):133–146. doi:10.1080/10659360600636196
Haenlein M, Kaplan AM (2004) A beginner’s guide to partial least squares analysis. Underst Stat 3(4):283–297. doi:10.1207/s15328031us0304_4
Eigenvector Research, Inc. (2014) PLS Toolbox 7.8.2. Eigenvector Research, Inc., Wenatchee
The MathWorks, Inc. (2014) MATLAB version 8.3. The MathWorks, Inc., Natick
Breiman L (2001) Random forests. Mach Learn 45(1):5–32. doi:10.1023/A:1010933404324
Gramatica P (2007) Principles of QSAR models validation: internal and external. QSAR Comb Sci 26(5):694–701. doi:10.1002/qsar.200610151
Sheridan RP (2012) Three useful dimensions for domain applicability in QSAR models using random forest. J Chem Inf Model 52(3):814–823. doi:10.1021/ci300004n
Topliss JG, Costello RJ (1972) Chance correlations in structure–activity studies using multiple regression analysis. J Med Chem 15(10):1066–1068. doi:10.1021/jm00280a017
Lin D, Xing B (2008) Adsorption of phenolic compounds by carbon nanotubes: role of aromaticity and substitution of hydroxyl groups. Environ Sci Technol 42(19):7254–7259. doi:10.1021/es801297u
Irving DL, Sinnott SB, Lindner AS (2004) Interaction of functionalized benezene molecules with carbon nanopores. Chem Phys Lett 389:96–100. doi:10.1016/j.cplett.2004.03.073
Tournas F, Latil S, Heggie MI, Charlier JC (2005) Pi stacking interaction between carbon nanotubes and organic molecules. Phys Rev B 72(7):075431. doi: 10.1103/PhysRevB.72.075431
Gianozzi P (2004) Comment on “Noncovalent functionalization of carbon nanotubes by aromatic organic molecules”. App Phys Lett 84(19):3936. doi:10.1063/1.1577381
Acknowledgements
The authors thank the National Science Foundation for financial support (NSF EPSCoR # 362492-190200-01\NSFEPS-0903787 and NSF CREST grant HRD # 1547754).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This paper belongs to Topical Collection 7th Conference on Modeling & Design of Molecular Materials in Trzebnica (MDMM 2016)
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 85 kb)
Rights and permissions
About this article
Cite this article
Watkins, M., Sizochenko, N., Moore, Q. et al. Chlorophenol sorption on multi-walled carbon nanotubes: DFT modeling and structure–property relationship analysis. J Mol Model 23, 39 (2017). https://doi.org/10.1007/s00894-016-3204-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3204-9