Abstract
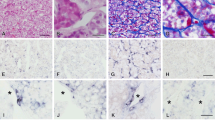
Extracellular matrix (ECM) is essential in tissue physiology and pathologic conditions such as tumorigenesis. ECM affects tumor cell behavior, proliferation, and metastasis. Pituitary adenomas vary in their clinical characteristics, including ECM deposition. However, the mechanism of desmoplasia in pituitary adenoma is not well understood. The present study focused on the principal component of ECM, collagen, and attempted to characterize collagen-producing cells in pituitary adenomas. Specimens of human pituitary adenomas and control pituitary were obtained during surgery. In situ hybridization for collagen I and III and immunohistochemistry for α-smooth muscle actin (a pericyte marker) and cytokeratin (an epithelial cell marker) were performed. The results showed that pericytes were the sole collagen-producing cells in control pituitary, while four types of collagen-producing cells were present in pituitary adenomas: pericytes, myofibroblasts, fibroblasts, and newly characterized “myoepithelial-like cells”. Azan staining showed that fibrous matrix deposition varied among pituitary adenomas and that the area of fibrosis was associated with the number and types of collagen-producing cells. These results suggest that changes in the number and type of collagen-producing cells influence ECM arrangement, which may in turn reflect pathologic characteristics in pituitary adenomas.






Similar content being viewed by others
References
Evans JJ, Chitcholtan K (2011) Extracellular matrix proteins in the anterior pituitary gland. Open Neuroendocr J 4:111–119
McNicol AM (2000) Tumors of the pituitary gland. In: Fletcher CDM (ed) Diagnostic histopathology of tumors. Churchill Livingstone, New York, pp 691–703
SojiT Herbert DC (1989) Intercellular communication between rat anterior pituitary cells. Anat Rec 224:523–533
Fujiwara K, Jindatip D, Kikuchi M, Yashiro T (2010) In situ hybridization reveals that type I and III collagens are produced by pericytes in the anterior pituitary gland of rats. Cell Tissue Res 342:491–495
Fujiwara K, Davaadash B, Yatabe M, Kikuchi M, Horiguchi K, Kusumoto K, Kouki T, Yashiro T (2008) Reduction of retinaldehyde dehydrogenase 1 expression and production in estrogen-induced prolactinoma of rat. Med Mol Morphol 41:126–131
Vannucci L (2015) Stroma as an active player in the development of the tumor microenvironment. Cancer Microenviron 8:159–166
Paez-Pereda M, Kuchenbauer F, Arzt E, Stalla GK (2005) Regulation of pituitary hormones and cell proliferation by components of the extracellular matrix. Braz J Med Biol Res 38:1487–1494
Yamada S, Fukuhara N, Horiguchi K, Yamaguchi-Okada M, Nishioka H, Takeshita A, Takeuchi Y, Ito J, Inoshita N (2014) Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases. J Neurosurg 121:1462–1473
Jarzembowski J, Lloyd R, McKeever P (2007) Type IV collagen immunostaining is a simple, reliable diagnostic tool for distinguishing between adenomatous and normal pituitary glands. Arch Pathol Lab Med 131:931–935
Wang H, Li WS, Shi DJ, Ye ZP, Tai F, He HY, Liang CF, Gong J, Guo Y (2008) Correlation of MMP(1) and TIMP(1) expression with pituitary adenoma fibrosis. J Neurooncol 90:151–156
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002) Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115:39–50
Chen WL, Huang CH, Chiou LL, Chen TH, Huang YY, Jiang CC, Lee HS, Dong CY (2010) Multiphoton imaging and quantitative analysis of collagen production by chondrogenic human mesenchymal stem cells cultured in chitosan scaffold. Tissue Eng 16:913–920
Lin SL, Kisseleva T, Brenner DA, Duffield JS (2008) Pericytes and perivascular fibroblasts are the primary source of collagen producing cells in obstructive fibrosis of the kidney. Am J Pathol 173:1617–1627
Sundberg C, Ivarsson M, Gerdin B, Rubin K (1996) Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest 74:452–466
Mack M, Yanagita M (2014) Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. doi:10.1038/ki.2014.287
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast. One function multiple origins. Am J Pathol 170:1807–1816
McAnulty RJ (2007) Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 39:666–671
Kato T, Mizuno S, Ito A (2014) A decrease in glomerular endothelial cells and endothelial-mesenchymal transition during glomerulosclerosis in the Tensin2-deficient mice (ICGN strain). Acta Histochem Cytochem 47:265–271
Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ (2005) Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia 10:261–272
Nagao T, Sato E, Inoue R, Oshiro H, Takahashi RH, Nagai T, Yoshida M, Suzuki F, Obikane H, Yamashina M, Matsubayashi J (2012) Immunohistochemical analysis of salivary gland tumors: application for surgical pathology practice. Acta Histochem Cytochem 45:269–282
Tajima S, Koda K (2015) Breast carcinoma with a predominant duct-replacing component and chondroid matrix production. Med Mol Morphol. doi:10.1007/s00795-015-0101-8
DiTommaso L, Pasquinelli G, Damiani S (2003) Smooth muscle cell differentiation in mammary stromo-epithelial lesions with evidence of a dual origin: stromal myofibroblasts and myoepithelial cells. Histopathology 42:448–456
Chen CZ, Raghunath M (2009) Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair 2:7. doi:10.1186/1755-1536-2-7
Abbass SAA, Asa SL, Ezzat S (1997) Altered expression of fibroblast growth factor receptors in human pituitary adenomas. J Clin Endocrinol Metab 82:1160–1166
Ren P, Scheithauer BW, Halper J (1994) Immunohistological localization of TGF alpha, EGF, IGF-I and TGF beta in the normal human pituitary gland. Endocr Pathol 5:40–48
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839
Tomita T (1997) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in pituitary adenomas: possible markers of neuroendocrine cells. Endocr Pathol 8:305–313
Acknowledgments
We thank Drs. Takehiro Tsukada, Morio Azuma, Motoshi Kikuchi (Jichi Medical University), Robert Yoshiyuki Osamura (International University of Health and Welfare, Mita Hospital) and Depicha Jindatip (Chulalongkorn University) for their helpful comments and suggestions. We are grateful to David Kipler, ELS (Supernatant Communications) for assistance in manuscript preparation. We also thank Dr. Naoki Murayama (Murayama Foundation) for his generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial assistance
This work was partly supported by promotional funds for the Keirin Race of the Japan Keirin Association.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The use of human tissues was approved by the Bioethics Committee for Epidemiologic Research of Jichi Medical University School of Medicine and the Ethics Committee of Toranomon Hospital, Japan, in accordance with established ethical standards.
Informed consent
All patients provided informed consent.
Rights and permissions
About this article
Cite this article
Tofrizal, A., Fujiwara, K., Yashiro, T. et al. Alterations of collagen-producing cells in human pituitary adenomas. Med Mol Morphol 49, 224–232 (2016). https://doi.org/10.1007/s00795-016-0140-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-016-0140-9