Abstract
Suicide is the second leading cause of death in the United States among individuals aged 10–24, and severe youth depression is often refractory to the current standards of care. Many studies have demonstrated the efficacy of ketamine in reducing depressive symptoms in adults with treatment-resistant mood disorders, though few studies utilizing ketamine in youth populations exist. This systematic review examines the current state of evidence for ketamine use in children with treatment-resistant mood disorders. We conducted a search utilizing two electronic databases for English-language studies investigating the therapeutic effects and side effect profile of ketamine in youth ≤ 19 years of age with a diagnosis of a treatment-resistant mood disorder. Analysis included subjects with treatment-resistant depression with and without psychotic features and with bipolar disorder. Primary outcome measures included the following scales: Montgomery–Asberg Depression Rating Scale, Children’s Depression Rating Scale, Children’s Depression Rating Scale Revised, Child Bipolar Questionnaire, Overt Aggression Scale, Yale–Brown Obsessive–Compulsive Scale, and Scale for Suicidal Ideation. Four published studies were identified that investigated therapeutic ketamine use in youth for the primary purpose of treating a treatment-resistant psychiatric disorder. Three additional studies that did not meet eligibility criteria were identified and discussed. Ketamine was shown in youth to generally improve depressive symptoms, decrease acute suicidality, and reduce mood lability, though a number of subjects remained resistant to its treatment. These findings substantiate the need for further longitudinal studies investigating ketamine’s long-term safety, its efficacy, and abuse potential in the youth.

Similar content being viewed by others
References
Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH et al (2019) Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr 206:256–267
National Institute of Mental Health (2019) Mental Health Information, Statistics, Major Depression
Bertha EA, Balázs J (2013) Subthreshold depression in adolescence: a systematic review. Eur Child Adolesc Psychiatry [Internet] 22(10):589–603
Clark MS, Jansen KL, Cloy JA (2012) Treatment of childhood and adolescent depression. Am Fam Physician [Internet] 86(5):442–448
Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A et al (2006) Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 45:1404–1411
March JS (2004) Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for Adolescents with Depression Study (TADS) randomized controlled trial. J Am Med Assoc 292:807–820
Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M et al (2008) Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA J Am Med Assoc 299:901–913
Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD et al (2010) Treatment of resistant depression in adolescents (TORDIA): week 24 outcomes. Am J Psychiatry [Internet] 167(7):782–791
Vitiello B, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller MB et al (2011) Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: a follow-up study of the TORDIA sample. J Clin Psychiatry 72:388–396
Berlim MT, Turecki G (2007) Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 52:46–54
Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O et al (1999) Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol [Internet] 9(1–2):83–91
Berlim MT, Turecki G (2007) What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol [Internet] 17(11):696–707
Werremeyer A (2014) Treatment-resistant depression. Ment Health Clin [Internet] 4(5):211–211
Birmaher B, Brent D (2007) Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry [Internet] 46(11):1503–1526
Mann JJ (2005) The medical management of depression. N Engl J Med [Internet] 353(17):1819–1834
Iiunma K, Arakawa Y, Takagi A, Kurata K, Ogihara T (1977) A simplified radioimmunoassay for serum aldosterone–non-extraction method with 125I-labeled aldosterone (author’s transl). Nihon Naibunpi Gakkai Zasshi [Internet] 53(6):797–809
Braconnier A, Le Coent R, Cohen D, DEROXADO Study Group (2003) Paroxetine versus clomipramine in adolescents with severe major depression: a double-blind, randomized, multicenter trial. J Am Acad Child Adolesc Psychiatry [Internet] 42(1):22–29
Maayan L, Correll CU (2011) Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol [Internet] 21(6):517–535
Ghaziuddin N, King CA, Naylor MW, Ghaziuddin M, Chaudhary N, Giordani B et al (1996) Electroconvulsive treatment in adolescents with pharmacotherapy-refractory depression. J Child Adolesc Psychopharmacol 6:259–271
Wachtel LE, Jaffe R, Kellner CH (2011) Electroconvulsive therapy for psychotropic-refractory bipolar affective disorder and severe self-injury and aggression in an 11-year-old autistic boy. Eur Child Adolesc Psychiatry [Internet] 20(3):147–152
Puffer CC, Wall CA, Huxsahl JE, Frye MA (2016) A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol 26:632–636
Walter G, McDonald A, Rey JM, Rosen A (2002) Medical student knowledge and attitudes regarding ECT prior to and after viewing ECT scenes from movies. J ECT 18:43–46
Taieb O, Flament M, Corcos M, Jeammet P, Basquin M, Mazet P et al (2001) Electroconvulsive therapy in adolescents with mood disorder: patients’ and parents’ attitudes. Psychiatry Res 104:183–190
Croarkin PE, Wall CA, McClintock SM, Kozel FA, Husain MM, Sampson SM (2010) The emerging role for repetitive transcranial magnetic stimulation in optimizing the treatment of adolescent depression. J ECT 26:323–329
Bloch Y, Grisaru N, Harel EV, Beitler G, Faivel N, Ratzoni G et al (2008) Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT 24:156–159
MacMaster FP, Croarkin PE, Wilkes TC, McLellan Q, Langevin LM, Jaworska N et al (2019) Repetitive transcranial magnetic stimulation in youth with treatment resistant major depression. Front Psychiatry [Internet] 10:170
Saluja G, Iachan R, Scheidt PC, Overpeck MD, Sun W, Giedd JN (2004) Prevalence of and risk factors for depressive symptoms among young adolescents. Arch Pediatr Adolesc Med [Internet] 158(8):760–765
Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE et al (1996) Childhood and adolescent depression: a review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry 35:1427–1439
Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J (1996) Childhood and adolescent depression: a review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry 35:1427–1439
Fergusson DM, Woodward LJ (2002) Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry 59:225–231
Vitiello B, Silva SG, Rohde P, Kratochvil CJ, Kennard BD, Reinecke MA et al (2009) Suicidal events in the treatment for adolescents with depression study (TADS). J Clin Psychiatry 70:741–747
Domino EF, Chodoff P, Corsenn G (1965) Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther [Internet] 6:279–291
Reich DL, Silvay G (1989) Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth [Internet] 36(2):186–197
United States Food and Drug Administration (1970) Ketalar NDA 016812 [Internet]. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=016812 (cited 7 Sep 2019)
Sadove MS, Shulman M, Hatano S, Fevold N (1971) Analgesic effects of ketamine administered in subdissociative doses. Anesth Analg [Internet] 50(3):452–457
Ihmsen H, Geisslinger G, Schüttler J (2001) Stereoselective pharmacokinetics of ketamine: R(−)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther [Internet] 70(5):431–438
Li L, Vlisides PE (2016) Ketamine: 50 years of modulating the mind. Front Hum Neurosci [Internet] 10:612
Reier CE (1971) Ketamine—"dissociative agent" or hallucinogen? N Engl J Med [Internet] 284(14):791–792
Jansen KL (2000) A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs [Internet] 32(4):419–433
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry [Internet] 47(4):351–354
Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Aan Het Rot M, Zarate CA, Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry [Internet] 72(7):537–547
Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF et al (2017) A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry [Internet] 74(4):399–405
Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI (2016) Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet [Internet] 55(9):1059–1077
Muller J, Pentyala S, Dilger J, Pentyala S (2016) Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol [Internet] 6(3):185–192
Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L et al (2013) Sub-anesthetic concentrations of (R, S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol [Internet] 698(1–3):228–234
Zeilhofer HU, Swandulla D, Geisslinger G, Brune K (1992) Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur J Pharmacol [Internet] 213(1):155–158
Chang L, Zhang K, Pu Y, Qu Y, Wang S-M, Xiong Z et al (2019) Comparison of antidepressant and side effects in mice after intranasal administration of (R, S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav [Internet] 181:53–59
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-I, Hashimoto K et al (2017) Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther [Internet] 361(1):9–16
Yang C, Shirayama Y, Zhang J, Ren Q, Yao W, Ma M et al (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry [Internet] 5:e632
Zhang J-C, Li S-X, Hashimoto K (2014) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav [Internet] 116:137–141
Persson J, Hasselström J, Maurset A, Oye I, Svensson JO, Almqvist O et al (2002) Pharmacokinetics and non-analgesic effects of S- and R-ketamines in healthy volunteers with normal and reduced metabolic capacity. Eur J Clin Pharmacol [Internet] 57(12):869–875
Leal GC, Bandeira ID, Correia-Melo FS, Telles M, Mello RP, Vieira F et al (2020) Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci [Internet]. https://doi.org/10.1007/s00406-020-01110-5
Correia-Melo FS, Leal GC, Carvalho MS, Jesus-Nunes AP, Ferreira CBN, Vieira F et al (2018) Comparative study of esketamine and racemic ketamine in treatment-resistant depression: protocol for a non-inferiority clinical trial. Medicine (Baltimore) [Internet] 97(38):e12414
United States Food and Drug Administration (2019) United States Food and Drug Administration: FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic [Internet]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (cited 18 Jan 2020)
Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM et al (2014) Off-label use of drugs in children. Pediatrics [Internet] 133(3):563–567. https://doi.org/10.1542/peds.2013-4060
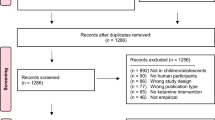
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev [Internet] 4:1
Veritas Health Innovation. Covidence systematic review software [Internet]. Veritas Health Innovation (cited 18 Jan 2020)
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry [Internet] 134:382–389
Poznanski EO, Cook SC, Carroll BJ (1979) A depression rating scale for children. Pediatrics [Internet] 64(4):442–450
Poznanski EO, Mokros HB (1996) Children’s Depression Rating Scale, Revised (CDRS-R), Los Angeles
Papolos D, Hennen J, Cockerham MS, Thode HC, Youngstrom EA (2006) The child bipolar questionnaire: a dimensional approach to screening for pediatric bipolar disorder. J Affect Disord [Internet] 95(1–3):149–158
Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D (1986) The overt aggression scale for the objective rating of verbal and physical aggression. Am J Psychiatry 143:35–39
Storch EA, Murphy TK, Adkins JW, Lewin AB, Geffken GR, Johns NB et al (2006) The children’s Yale–Brown obsessive–compulsive scale: psychometric properties of child- and parent-report formats. J Anxiety Disord [Internet] 20(8):1055–1070
Beck AT, Kovacs M, Weissman A (1979) Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol [Internet] 47(2):343–352
Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress [Internet] 11(1):125–136
Cullen KR, Amatya P, Roback MG, Albott CS, Westlund Schreiner M, Ren Y et al (2018) Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol [Internet] 28(7):437–444
Papolos DF, Teicher MH, Faedda GL, Murphy P, Mattis S (2013) Clinical experience using intranasal ketamine in the treatment of pediatric bipolar disorder/fear of harm phenotype. J Affect Disord [Internet] 147(1–3):431–436
Zarrinnegar P, Kothari J, Cheng K (2019) Successful use of ketamine for the treatment of psychotic depression in a teenager. J Child Adolesc Psychopharmacol [Internet] 29:472–473
Dwyer JB, Beyer C, Wilkinson ST, Ostroff RB, Qayyum Z, Bloch MH (2017) Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry [Internet] 56(4):352–354
Castellanos A, Moleiro F, Guerrero J, Awaad MI, Myerburg RJ (1997) Intermittent parasystole with exit block. J Electrocardiol [Internet] 30(4):331–335
Donoghue AC, Roback MG, Cullen KR (2015) Remission from behavioral dysregulation in a child with PTSD after receiving procedural ketamine. Pediatrics [Internet] 136(3):e694–e696. https://doi.org/10.1542/peds.2014-4152
Papolos D, Frei M, Rossignol D, Mattis S, Hernandez-Garcia LC, Teicher MH (2018) Clinical experience using intranasal ketamine in the longitudinal treatment of juvenile bipolar disorder with fear of harm phenotype. J Affect Disord [Internet] 225:545–551
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. Revision 4th edition with text, editor. American Psychiatric Press, Washington, DC, pp 1–2
Beck AT (1988) Beck Hopelessness Scale, San Antonio
Weber G, Yao J, Binns S, Namkoong S (2018) Case report of subanesthetic intravenous ketamine infusion for the treatment of neuropathic pain and depression with suicidal features in a pediatric patient. Case Rep Anesthesiol [Internet] 2018:9375910
Guy W (1976) ECDEU Assessment Manual for Psychopharmacology. U.S. Government Printing Office, Rockville
Mason KP, Padua H, Fontaine PJ, Zurakowski D (2009) Radiologist-supervised ketamine sedation for solid organ biopsies in children and adolescents. AJR Am J Roentgenol [Internet] 192(5):1261–1265
Sheehy KA, Muller EA, Lippold C, Nouraie M, Finkel JC, Quezado ZMN (2015) Subanesthetic ketamine infusions for the treatment of children and adolescents with chronic pain: a longitudinal study. BMC Pediatr [Internet] 15:198
Minoshima R, Kosugi S, Nishimura D, Ihara N, Seki H, Yamada T et al (2015) Intra- and postoperative low-dose ketamine for adolescent idiopathic scoliosis surgery: a randomized controlled trial. Acta Anaesthesiol Scand [Internet] 59(10):1260–1268
Zempsky WT, Loiselle KA, Corsi JM, Hagstrom JN (2010) Use of low-dose ketamine infusion for pediatric patients with sickle cell disease-related pain: a case series. Clin J Pain [Internet] 26(2):163–167
Finkel JC, Pestieau SR, Quezado ZMN (2007) Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain [Internet] 8(6):515–521
Allen JY, Macias CG (2005) The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Ann Emerg Med [Internet] 46(1):43–50
Dobbing J, Sands J (1973) Quantitative growth and development of human brain. Arch Dis Child [Internet] 48(10):757–767
Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev [Internet] 3(1):79–83
Hayashi H, Dikkes P, Soriano SG (2002) Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr Anaesth [Internet] 12(9):770–774
Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U et al (2001) Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol [Internet] 62(4):401–405
Liu F, Paule MG, Ali S, Wang C (2011) Ketamine-induced neurotoxicity and changes in gene expression in the developing rat brain. Curr Neuropharmacol [Internet] 9(1):256–261
Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W et al (2017) The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast [Internet] 2017:6871089
Wang J, Zhou M, Wang X, Yang X, Wang M, Zhang C et al (2014) Impact of ketamine on learning and memory function, neuronal apoptosis and its potential association with miR-214 and PTEN in adolescent rats. PLoS One [Internet] 9(6):e99855
Yan J, Jiang H (2014) Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain. J Neurosurg Anesthesiol [Internet] 26(2):155–160
Bates MLS, Trujillo KA (2019) Long-lasting effects of repeated ketamine administration in adult and adolescent rats. Behav Brain Res [Internet] 369:111928
Onaolapo AY, Ayeni OJ, Ogundeji MO, Ajao A, Onaolapo OJ, Owolabi AR (2019) Subchronic ketamine alters behaviour, metabolic indices and brain morphology in adolescent rats: involvement of oxidative stress, glutamate toxicity and caspase-3-mediated apoptosis. J Chem Neuroanat [Internet] 96:22–33
Hijazi Y, Boulieu R (2002) Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos [Internet] 30(7):853–858
Martignoni M, Groothuis GMM, de Kanter R (2006) Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol [Internet] 2(6):875–894
Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK et al (2013) Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiatry [Internet] 74(10):750–759
Lee K-H, Yeh Y-C, Yang P-C, Lin H-C, Wang P-W, Liu T-L et al (2012) Individual and peer factors associated with ketamine use among adolescents in Taiwan. Eur Child Adolesc Psychiatry [Internet] 21(10):553–558
Shepard RD, Langlois LD, Browne CA, Berenji A, Lucki I, Nugent FS (2018) Ketamine reverses lateral habenula neuronal dysfunction and behavioral immobility in the forced swim test following maternal deprivation in late adolescent rats. Front Synaptic Neurosci [Internet] 10:39
Naughton M, Clarke G, O’Leary OF, Cryan JF, Dinan TG (2014) A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord [Internet] 156:24–35
Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH (2016) Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety [Internet] 33(8):689–697
Kraus C, Wasserman D, Henter ID, Acevedo-Diaz E, Kadriu B, Zarate CA (2019) The influence of ketamine on drug discovery in depression. Drug Discov Today [Internet] 24(10):2033–2043
Croarkin PE, Nakonezny PA, Husain MM, Melton T, Buyukdura JS, Kennard BD et al (2013) Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry [Internet] 70(3):291–299
Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A et al (2014) Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol [Internet] 17(2):331–336
Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y (2008) Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol [Internet] 11(8):1047–1061
Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN (2010) Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog Neuropsychopharmacol Biol Psychiatry [Internet] 34(4):645–651
Pandey GN, Rizavi HS, Dwivedi Y, Pavuluri MN (2008) Brain-derived neurotrophic factor gene expression in pediatric bipolar disorder: effects of treatment and clinical response. J Am Acad Child Adolesc Psychiatry [Internet] 47(9):1077–1085
Pacheco D da F, Romero TRL, Duarte IDG (2014) Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res [Internet] 1562:69–75
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H et al (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry [Internet] 175(12):1205–1215
Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ et al (2014) Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety [Internet] 31(4):335–343
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM et al (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry [Internet] 170(10):1134–1142
Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R et al (2015) Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev [Internet] 9:CD011612
Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol [Internet] 30(3):152–163
Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry [Internet] 5(1):65–78
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S et al (2014) Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry [Internet] 71(6):681–688
Mathew SJ, Wilkinson ST, Altinay M, Asghar-Ali A, Chang LC, Collins KA et al (2019) Electroconvulsive therapy (ECT) vs. ketamine in patients with treatment-resistant depression: the ELEKT-D study protocol. Contemp Clin Trials [Internet] 77:19–26
AACAP Psychopharmacology Committee (2018) AACAP Psychopharmacology Committee Statement on Ketamine
Turner EH (2019) Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry [Internet] 6(12):977–979
Singh JB, Daly EJ, Mathews M, Fedgchin M, Popova V, Hough D et al (2020) Approval of esketamine for treatment-resistant depression. Lancet Psychiatry [Internet] 7(3):232–235
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No statistical consultants were employed in this study. No support for this study was received from any grant, funding source, or commercial interest.
Rights and permissions
About this article
Cite this article
Kim, S., Rush, B.S. & Rice, T.R. A systematic review of therapeutic ketamine use in children and adolescents with treatment-resistant mood disorders. Eur Child Adolesc Psychiatry 30, 1485–1501 (2021). https://doi.org/10.1007/s00787-020-01542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01542-3