Abstract
Nutrition plays an important role in neurodevelopment. This insight has led to increasing research into the efficacy of nutrition-related interventions for treating neurodevelopmental disorders. This review discusses an elimination diet as a treatment for attention deficit hyperactivity disorder and autism spectrum disorder, with a focus on the efficacy of the food additives exclusion diet, gluten-free/casein-free diet and oligoantigenic diet. Furthermore, we discuss the potential mechanisms of elimination diets’ effects in these neurodevelopmental disorders. The main candidate mechanism is the microbiome–gut–brain axis possibly involving complex interactions between multiple systems, including the metabolic, immune, endocrine, and neural system. We conclude with practical implications and future directions into the investigation of an elimination diet’s efficacy in the treatment of attention deficit hyperactivity disorder and autism spectrum disorder.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) are early onset neurodevelopmental disorders that may persist into adulthood. Both ASD and ADHD are umbrella terms that cover heterogeneity of behavioral abnormalities. ADHD is characterized by inattentive, hyperactive and impulsive behavior, whereas the key symptoms of ASD include social deficits, communication deficits and stereotypical behavior [1]. Despite the differences in the core behavioral symptoms between ASD and ADHD, there are indications for an overlap between the disorders. Indeed, research indicates that these disorders are highly comorbid [2–7]. This overlap could be explained by a shared etiology between ADHD and ASD disorders; for instance, genetic studies have demonstrated common genetic etiology for these disorders [8, 9]. Although the etiology of ASD and ADHD remains largely unknown, a complex interaction of genetic and environmental factors is thought to contribute to the development of ASD and ADHD [2, 10, 11].
One of the potential environmental risk factors for neurodevelopmental disorders is diet [12]. Nutrition has an impact on neurodevelopment, cognition, and behavior, and could therefore play an important role in neurodevelopmental disorders [13–15]. This insight, together with the lack of effective treatments for core ASD symptoms and the concerns about the safety of pharmacological treatments in ADHD [16], have led to increasing research into the efficacy of nutrition-related interventions as additional or alternative (non-pharmacological) treatments for these disorders. The dietary interventions that have been subjected to clinical trials include various forms of elimination diets and supplementation interventions [17–20]. In this review, we provide an overview of the literature with regard to the most common forms of elimination diets and their efficacy in ADHD and ASD. Furthermore, we discuss the potential mechanisms of elimination diets’ effects in both ADHD and ASD. Finally, we conclude with the practical implications and future directions into the investigation of elimination diets’ efficacy in the treatment of these early onset neurodevelopmental disorders.
Elimination diets in ASD and ADHD
At first, the concept of an elimination diet was introduced to diagnose and treat food allergies [21]. The main reason for applying an elimination diet is to find out which foods are causing or aggravating physical adverse reactions. Later this link of food with adverse physical adverse reactions has been extended to a connection with neurobehavioral symptoms [22, 23]. Thus, in this later view, behavioral reactions are seen as possible adverse reactions to food as well. In the domain of psychiatry, it is therefore believed that elimination diets can be used to identify foods that contribute to mental disorders and associated behavioral and cognitive disturbances.
Elimination diets come in different forms and vary in their strictness and food items that are being eliminated. Interestingly, the forms of elimination diets that are being investigated differ between ASD versus ADHD. In ASD, the gluten-free and/or casein-free (GFCF) diet has been mainly investigated, whereas in ADHD, clinical trials examined the effects of food additives exclusion diets and the oligoantigentic diets. These differences in focus between the elimination diets that are applied in the two disorders are due to the different origins of the research in both fields. In the following sections, the origins, rationale, and the general principles of the diets are being described, followed by the degree of evidence with regard to the efficacy. In Table 1, an overview is provided for several characteristics of the respective diets, including the excluded food items, main target group, efficacy, and nutritional elements that would require attention in case of long-term exclusion to avoid deficiencies.
Gluten-free diet and/or casein-free diet in ASD
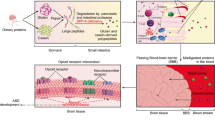
The focus on GFCF diets in ASD originates from the evidence of comorbid gastrointestinal tract problems in patients with ASD [24–26]. The explanation for increased gastrointestinal problems in ASD remains unclear. One explanation is that gastrointestinal tract problems in patients with ASD might be caused by increased intestinal permeability, the so-called leaky gut, which has indeed been demonstrated in children with ASD compared to healthy controls [27–29]. According to the ‘opioid excess theory’, digestion of gluten and casein produces peptides with an opioid activity that can enter the bloodstream when the gut permeability is high [30, 31]. These neuroactive peptides can in turn bind to opioid receptors and are therefore speculated to affect processes in the central nervous system [32].
The idea for the gluten-free diet involves examination of the effects of removing all the food items containing gluten, a mixture of proteins found in wheat, oats, barley, or rye. Thus, all products made with these cereals are excluded from the diet and replaced with special gluten-free version of the common food items. In some cases, the gluten-free diet is combined with a casein-free diet. Casein is a peptide commonly found in milk. Thus, a casein-free diet involves avoiding the intake of milk and dairy products. After a period of elimination, products containing gluten or casein can be reintroduced to test whether it is contributing to the symptoms.
Food additives exclusion diet in ADHD
As discussed above, the elimination diet was originally proposed as a tool for treatment of food allergies [21]. Feingold, an American pediatrician and allergist, was the first who suggested that allergy to food additives, such as artificial flavors and colors, as well as naturally occurring salicylates could lead to ADHD symptoms [33]. He based this on observations that aspirin, which contains salicylates, could not only lead to allergic reactions, but also to an increase of hyperkinetic behavior in some patients. This observation has set the stage for the development of various food additives exclusion diets, which typically involves the removal of, for instance, artificial food colors, flavors, fragrances, preservatives, and sweeteners. Sometimes, this diet is part of a broader elimination diet, such as the Feingold diet or Kaiser Permanente diet (http://feingold.org/), which involves the removal of both natural and artificial salicylates. The intervention usually investigates the effect over periods of a week or longer. Food challenges that include food additives can be used after this period to examine the acute immediate effects of the challenge. The hypothesis is that ADHD could be influenced by either allergenic or pharmacologic mechanisms related to levels of salicylates and salicylates-like substances.
Oligoantigenic diet in ADHD
In oligoantigenic diets, the focus is on eliminating suspected high allergenic food products rather than eliminating artificial colors, flavors, and preservatives specifically. An oligoantigenic diet intervention, also known as a restricted elimination diet or hypoallergenic diet, involves the testin657g of an individually constructed restricted elimination diet. The focus is on eliminating foods that are often found to be highly allergenic, such as cow’s milk, cheese, egg, chocolate, and nuts. Oligoantigenic diets come in different forms and vary in their strictness. The oligoantigenic diet typically involves an elimination phase (usually two to five weeks) in which the specific food items are excluded completely. The food items in the elimination phase could, for instance, consist of only a few hypoallergenic foods such as rice, turkey, lettuce, pears, and water [34]. If the patient reacts by a substantial decrease of symptoms indicating ‘food sensitivity’, a reintroduction phase, which could take as long as eighteen months, could be applied to find out what specific food items trigger the symptoms. Thus, an elimination diet can be regarded as a ‘diagnostic’ tool for determining whether specific foods cause adverse physical and/or behavioral reactions.
Evidence for elimination diets’ effects on ADHD and ASD symptoms
Gluten-free/casein-free diet in children with ASD
There is increasing research into the effectiveness of the GFCF diet in children diagnosed with ASD. Several systematic reviews of GFCF diet studies in ASD have appeared [19, 35–40]. However, the number of high quality RCTs is too small for drawing firm conclusions at this point and there are no meta-analyses available. Overall, the findings with regard to GFCF diet’s effect in ASD are mixed. Several studies suggest no effect of the GFCF diet’s effect in ASD [41–43]. For instance, a recent study testing the effects of a GFCF diet in children with ASD (n = 30) involving a double-blind placebo-controlled challenge phase did not demonstrate positive effects on measures of physiological functioning, behavior problems, or ASD symptoms [41]. However, children with known gastrointestinal disorder were excluded, which could have weakened any potential effects [41]. In fact, there is research suggesting that gastrointestinal issues might go together with ASD behavioral symptoms and reactions to gluten and casein [46, 47]. Two RCTs investigated GFCF diet’s effect in ASD by following children with ASD (n = 10 and n = 72) for 1 year [44, 45]. The data demonstrated positive effects of the GFCF diet on ASD trait measures. However, the outcome measure was based on unblinded caregivers’ reports and the studies did not control for concomitant treatments that the children may have received while being enrolled in the studies. Furthermore, the attrition rate was nearly a quarter in one of the studies, which was not taken into account in the analysis.
Elimination diets in children with ADHD
The effectiveness of the food additives exclusion diet as treatment for ADHD has been investigated in a number of studies [17, 18, 48]. Only some studies additionally eliminated food items containing natural salicylates as part of a broader diet, such as the Feingold diet or Kaiser Permanente diet [49, 50]. A meta-analysis with a focus on the effectiveness of such elimination diets in ADHD across twenty studies including 794 participants found a small effect size based on parent reports, 0.18, that decreased to 0.12 when taking into account possible publication bias [51]. The effects based on teachers’ reports and observer measures were not significant. However, pooling the data of a limited number of high quality studies for analyses in this meta-analysis demonstrated small effects on teacher ratings and neurocognitive attentional tests. In another meta-analysis, focusing exclusively on ADHD outcomes and more critically addressed assessment blinding issues, eight studies including 294 participants reported an effect size of 0.32 for the most proximal assessment and 0.42 for the probably blinded assessments [18]. It is important to note though that the effects of this meta-analysis may be limited to children with ADHD who have sensitivity to food colors, as the participants were often preselected on the basis of a suspected sensitivity to food colors during the elimination phase of the diet. Furthermore, rather than an increase in the effect size, additional analysis using only trials with low/no comedication to exclude the impact of effective medication led to a reduction in the effect size to a non-significant level [18]. Stevenson et al. reported that high quality studies showed an effect size of 0.21 and 0.22 of food color elimination; however these studies were not restricted to children diagnosed with ADHD [17]. It has been suggested that the effect of food color elimination on behavior is probably not limited to children with a diagnosis of ADHD, but rather also applies to hyperactive behavior in children more generally [48].
With regard to oligoantigenic diets, the effectiveness of the elimination phase has been demonstrated in several randomized clinical trials with patients with ADHD. An effect size of 0.29 has been reported in a meta-analysis across six controlled trials including 195 participants; it was concluded that about one third of the children with ADHD show an excellent (>40% symptom reduction) response [51]. This meta-analysis excluded two studies by Pelsser et al. with an outlier effect size and the use of non-blinded ratings [34, 52]. Another meta-analysis including these two studies estimated an effect size of 1.48 that, however, dropped substantially to 0.51 (95% confidence interval −0.02 to 1.04) when probably blinded raters were used [18].
Taken together, it remains inconclusive whether elimination diets are effective as a treatment for children with ASD and ADHD. So far, the evidence for GFCF diets as an effective treatment for children with ASD is weak, but it might be a beneficial intervention for a subset of children with ASD, particularly for those with comorbid gastrointestinal problems. The observed effects for food additives exclusion diets in children with ADHD are small, and may not be specific for children with ADHD. Although a few select studies using most proximal assessment demonstrated large effects of oligoantigenic diets in children with ADHD [34, 52], an overall small effect of this diet was shown by other studies using probably blinded assessments. Furthermore, it remains unknown whether children will have a consolidated diet after the elimination phase of the oligoantigenic diet. This question calls for studies that include food challenges or a reintroduction phase in the study design. Double-blind placebo-controlled challenges or reintroduction phases after positive reactions to eliminations are useful for further investigations to test the effects of the food items in a systematic and controlled way. Studies that assess the long-term effectiveness are necessary to draw conclusions about elimination diet’s effects in children with ADHD and ASD. Finally, contrary to most aforementioned work, future studies should adopt the gold standard for clinical trials, i.e., high quality randomized controlled trials with blinded raters for the primary outcome.
Mechanisms of elimination diets’ effect
Food allergy and hypersensitivity
A typical food allergic reaction is mediated by specific immunoglobulin (Ig) antibodies, IgE. Such IgE-mediated allergic reaction has an immediate onset of symptoms within several minutes to several hours after contact with the allergen. The dietary interventions in children with ASD have mainly focused on the elimination of gluten and/or casein and were based on the hypothesis that children with ASD have an increased risk of immune responses to these substances. Although no association between gluten sensitivity and ASD has been reported based on immune markers [24], serum levels of immunoglobulin antibodies IgA, IgG, and IgM specific for milk-derived allergens and total IgE were increased in children with ASD compared with controls [53]. In addition, stimulation of peripheral blood mononuclear cells of children with ASD using milk-derived allergens produced more pro-inflammatory cytokines compared to controls [54]. Overall, there is increasing evidence that dysregulation of the immune system plays an important role in ASD. For instance, it has been shown that children with ASD have reduced regulatory T cell response, indicating reduced tolerance to antigens in these children [55]. ASD has also been associated with increased levels of pro-inflammatory cytokines in the blood, and decreased levels of anti-inflammatory cytokines [56, 57]. Furthermore, deficiencies in the complement system, which plays an important role in promoting immune cells to clear microbes, infected cells, and cellular debris, have also been demonstrated in ASD [58, 59]. Taken together, there is some indication that ASD is linked to food allergy or ‘hypersensitivity’, which could provide an explanation for the potential efficacy of GFCS diets in the treatment of ASD.
The aim of food additives exclusion diet and oligoantigenic diet is to eliminate foods from the diet that trigger adverse physical, allergic reactions. Thus, the concept of these diets is based on a link between ADHD and allergic reactions to food. It has been shown that children with ADHD have an increased risk of developing allergies and vice versa [60, 61]. However, so far there is no convincing evidence for food allergy or hypersensitivity to be involved in ADHD. For instance, no differences were found between patients with ADHD and healthy controls on a skin prick test to food allergens [62]. Moreover, hyperactive children who responded to a challenge test with allergenic food products did not demonstrate positive skin prick tests [63]. These findings may suggest that ADHD is associated with a non-IgE-mediated reaction to food. It has been hypothesized that determination of IgG may be helpful in these cases, such that foods that induce high IgG-levels would lead to a worsening of symptoms, while foods that induce low IgG-levels would not. However, there is no evidence for IgG as a potential marker at this point. In a previous study, a challenge test with allergenic foods has led to relapse of ADHD symptoms independently of influences on IgG blood levels, thus questioning the role of IgG-mediated mechanisms [34]. Despite the lack of clear serological markers indicating food allergy in ADHD, future studies should further investigate this open question and examine whether food allergy or hypersensitivity could play a role in ADHD and mediate elimination diets’ effects, particularly given the previous findings suggesting a link between ADHD and allergies [60, 61, 64].
Microbiome–gut–brain axis
Diet and the role of the microbiome in health and disease
The intestinal microbial flora is involved in appetite regulation, energy utilization, digestion and absorption of nutrients [65, 66]. Additionally, our microbiome plays a crucial role in health and disease by influencing immune function, drug metabolism and protection against pathogens [67–69]. Disruption of the microbiome composition or dysbiosis has been associated with several human diseases, including inflammatory bowel diseases, cancer, obesity, metabolic syndrome and neurological disorders [70–77]. Moreover, the role of microbiome in multiple complex disorders is supported by evidence from safe treatment using fecal transplants in inflammatory bowel diseases, but also in disorders less directly linked to the gut, like multiple sclerosis and chronic fatigue syndrome [78]. Current evidence from animal studies convincingly shows that the composition of the microbiome has an impact that is even beyond disease, (co-)determining mood, stress response, and several aspects of behavior [79–82]. Diet is an important determinant of gut microbiota composition and functioning that is strongly linked with psychopathological outcomes [83–85]. Together, these findings suggest that there is a link between diet, the composition of the gut microbiome and mental disorders.
Multiple pathways in the microbiome–gut–brain axis
The effects of the microbiome on brain function, behavior and diseases can be mediated through different pathways of the so-called microbiome–gut–brain axis, involving neural, as well as metabolic, immune and endocrine mechanisms [79, 86–88]. A number of reviews have provided a thorough overview of these complex interactions [79, 86–88]. In brief, at least three pathways have been suggested to link the gut microbiome with brain function. First, the brain and the intestinal system are connected via the vagus nerve, a major nerve of the parasympathetic nervous system. The microbiome could influence brain function by innervation of the vagus nerve. The vagus nerve seems to differentiate between non-pathogenic and pathogenic bacteria and mediate signals that can induce anxiogenic and anxiolytic effects depending on the nature of the stimulus. These effects potentially involve immunomodulatory mechanisms [89]. For example, in a rodent study, it has been shown that ingestion of the Lactobacillus rhamnosus reduced stress-induced corticosterone and anxiety- and depression-related behavior [90]. These effects were accompanied by alterations in central γ-aminobutyric acid (GABA) receptor expression, which has often been associated with anxiety, depression, and bowel disorders [91]. Crucially, these neurochemical and behavioral effects were not found in vagotomized mice, suggesting that the vagus nerve plays a mediating role in these effects and functions as a communication pathway between gut bacteria and the brain [90]. Second, the microbiome could influence brain function via interactions with the immune system [92, 93]. Bacterial species can affect the immune system effects through the production of immune-regulatory metabolites, such as short-chain fatty acids (SCFAs) [94–96]. Alterations in regulation of neuroactive bacterial products or metabolites, can alter gene expression, influence the immune system, and interact with nerve cells by stimulating the sympathetic nervous system [97–99]. Third, the composition of the microbiome might influence the levels of neurotransmitters and thereby processes in the brain. For instance, bacterial strains can influence the synthesis and thereby the release of serotonin, a key neurotransmitter in the gut–brain connection that plays an important role in gastrointestinal and neurobehavioral regulations [100–102]. A recent rodent study has shown evidence that indigenous spore forming bacteria are able to stimulate serotonin-producing colonic enterochromaffin cells and modulate gastrointestinal motility via metabolic signaling with specific metabolites. Furthermore, an increase of the particular metabolites was associated with increases in colonic and serum serotonin in the mice, suggesting an important role of the spore forming bacteria in the regulation of serotonin levels and serotonin-related processes in the host. In addition to serotonin and SCFAs, gut bacteria are also capable of producing an array of other neuroactive and immunomodulatory compounds, including dopamine, GABA, histamine, and acetylcholine [103–106].
The role of the microbiome–gut–brain axis in neurodevelopment
The development of the microbiome–gut–brain axis happens early in life. The bacterial colonization is a postnatal event, which commences at birth. After one-to-three years of age, a complex adult-like microbiome is evident and stable [107–109]. Parallels can be drawn between the development of the microbiome and the development of the central nervous system suggesting that critical windows in neurodevelopment might be linked to critical periods in the colonization of the microbiome [86]. It has therefore been suggested that the microbiome–gut–brain axis plays an important role in neurodevelopmental disorders [86, 88]. However, the precise factors in the microbial composition that may cause disruptions in neurodevelopmental processes are not yet identified. Nevertheless, there is some evidence in support for this idea: for instance, it has been shown that germ-free mice have an exaggerated HPA-axis response to an external stressor, while this effect was reversed by administration of the feces from specific pathogen-free mice with normal gut microbiota at early stage of development, but not at later stage [110]. This suggests that exposure to endogenous microbiota at an early developmental stage is required for the HPA system to become fully susceptible to inhibitory neural regulation. More generally, these findings suggest that the microbiome may contribute to critical windows in neurodevelopment, and it is relevant to consider the role of the microbiome in neurodevelopmental disorders, such as ASD and ADHD.
The role of the microbiome–gut–brain axis in ASD
In individuals with ASD, altered composition of the intestinal microbiota has been demonstrated [111–115]. For instance, studies in ASD have found Clostridium or Desulfovibrio bacteria clusters over-represented in children with gastrointestinal complaints and ASD as compared to children with similar gastrointestinal complaints but typical neurobehavioral development [113, 116, 117]. Although the results with regard to the specific species of microbes that are altered in individuals with ASD versus controls are inconsistent across studies, alterations in the Clostridium species have been reported across several studies in individuals with ASD [111, 113, 117]. Clinical improvement has additionally been reported anecdotally in children with ASD who develop fever [118], receive oral antibiotics [119], or ingest probiotics [120], all of which likely alter the gut microbiome. Using an animal model of ASD with gastrointestinal and neurological symptoms, the supply of Bacteroides fragilis, commensal intestinal bacteria, has been shown to reduce the gastrointestinal symptoms, improve the intestinal barrier permeability, and reduce ASD-related behavioral disturbances [121].
As discussed previously, other theories with regard to the mechanism underlying elimination diet’s effects in ASD relate to metabolic deficiencies (though not excluding a role of the microbiome). It has been postulated that ASD is the result of a metabolic disorder in which opioid peptides produced through metabolism of gluten and casein pass through an abnormally high permeable intestinal membrane (‘opioid excess theory’ or ‘leaky gut theory’) [27–31]. These neuroactive peptides can then enter the blood stream and exert an effect on neurotransmission through binding with opioid receptors. Indeed, a higher permeability of the gut has been found in children with ASD compared to the gut permeability of healthy controls [27]. However, so far experiments have failed to detect any abnormal high levels of opioid peptides in children with ASD [122]. Another potential theory relates to oxidative stress and sulfur metabolic deficiencies. In a vicious circle, sulfur metabolic deficiencies might influence the bacterial composition and bacterial products leading to elevated oxidative stress in individuals. Together, bacterial products, oxidative stress and dietary allergens could lead to increased intestinal permeability [123]. Neuroinflammation, affecting the protective blood–brain-barrier capacity, as a result of increased gastrointestinal permeability could contribute to ASD. Therefore, a dietary treatment that is able to restore the gut permeability can be effective as a treatment for children with ASD [123–125].
The role of the microbiome–gut–brain axis in ADHD
Although direct evidence for a role of changed microbiome–gut–brain interaction is currently lacking in ADHD, converging evidence suggests that similar alterations in microbiome–gut–brain interaction can contribute to ADHD symptoms as well [64, 126]. Our group has recent unpublished data showing that gut microbiome is altered in ADHD. More specifically, increases in the genus Bifidobacterium in ADHD versus controls contributed to the increased metabolic function of cyclohexadienyl dehydratase, an enzyme that is involved in the synthesis of the essential amino acid and dopamine precursor, phenylalanine. Furthermore, in the same study, we demonstrated that these metabolic changes were related to decreased ventral striatal signals to reward anticipation, a neural hallmark of ADHD [127, 128]. These findings could be an important first step towards a better understanding of the relationship between alterations in the microbiome–gut–brain axis and ADHD symptoms. Further investigations are necessary to establish this relationship, and the role of diet.
Family relationships and structure
Since strict parental supervision is necessary for the application of an elimination diet, one could argue that elimination diets’ effects might be explained by concomitant adaptations in parental behavioral strategies and care. It is known that parents of children with ADHD and ASD use different parenting structure and have a different relationship with their children [129–131]. Furthermore, consistent parenting and positive parent–child interactions are associated with improvements of child behavior [132]. Although it should be noted that for ADHD, behavioral interventions mainly have shown positive effects on other outcomes that are associated with the disorder rather than on the primary symptoms of the disorder [18, 133]. Nevertheless, one could argue that there is a possibility that elimination diets’ effects as reflected by behavioral improvements might be mediated by the change in parenting structure required for the application of the elimination diet, rather than by a direct result of dietary changes per se. However, this hypothesis has not been supported by any evidence so far. In a previous study, it was demonstrated that families of children with ADHD, who were motivated to follow an elimination diet, already showed a good family environment, i.e., no difference compared to families of health controls. Moreover, the elimination diet did not affect the family relationships and structure [134]. More research is necessary to investigate whether family relationships, family structure, and other psychosocial variables may play a potential role in elimination diet’s effects.
In sum, the mechanism underlying the effectiveness of the elimination diet is yet unknown. The microbiome–gut–brain axis is the main candidate mechanism and could potentially involve interactions between allergic reactions, intestinal permeability, oxidative stress, and alterations in microbiome composition and function. Characterization of the direct and indirect pathways of the microbiome–gut–brain connection is important, as it can ultimately inform clinicians as to potential markers and targets for (preventative) treatment. Furthermore, this knowledge acquisition is an important step in the development of novel nutritional/therapeutic interventions tailored at the individual patient. More knowledge about the mechanisms underlying elimination diets’ effects in ADHD and ASD is needed.
Clinical implications and future research
The GFCF diet might be beneficial for children with ASD and food intolerance/allergy or underlying gastrointestinal disease. However, the evidence for the effectiveness of GFCF diets in children with ASD is weak and thus these diets cannot be generally recommended as a treatment for children with ASD. Yet, a GFCF diet is a commonly applied intervention in children with ASD in practice. Many parents may feel it is better to apply any available treatment when searching for treatment for their child with ASD as therapies for treating core symptoms of ASD are limited. However, using a GFCF diet may not be without harm (e.g., stigmatization, diversion of treatment resources, nutritional deficiency) [135]. Hence, until there is conclusive evidence for the benefits of GFCF diets for children with ASD, it is the task of health professionals and researchers to communicate with caregivers and inform them about the costs and potential adverse consequences.
Benefits of food additives exclusion diets have been suggested in children diagnosed with ADHD. However, the observed effects are small, which makes food additives exclusion diet not qualified as stand-alone treatment. Nevertheless, given the positive, albeit small effects of this intervention in children with ADHD as well as in children from the general population, and the fact that food additives do not provide any health benefits, it is recommended that children preventatively minimize consumption of processed food products with these ingredients. Moreover, from a public health perspective, food industry might change its policy and lower or even avoid adding unnecessary additives to food.
With regard to oligoantigenic diets, if applied under close supervision, these interventions could be valuable instruments to assess whether ADHD is triggered by food. However, the efficacy of this diet as a treatment for ADHD remains to be investigated as there are no studies yet that have demonstrated long-term beneficial effects of this treatment after consolidating the diet. Our research group is currently conducting a large clinical trial to assess the long-term beneficial effects of an oligoantigenic diet in children with ADHD.
Previous work has shown that only a subset of the children with ADHD responds to an oligoantigenic diet [34]. However, the characteristics of responders versus non-responders remain to be determined in future work. In the application of an elimination diet, it is important to take the individual differences in treatment response into account. First observations from our ongoing randomized controlled trial (www.project-trace.nl) suggest that some children might not improve on the primary symptoms, but rather on symptoms that usually accompany the disorder, such as emotion regulation problems (e.g., irritability) and physical symptoms (e.g., intestinal disturbances). These observations might suggest that the effects of an elimination diet are not per se symptom/diagnosis specific and it could be important to monitor changes in different domains that are relevant for the well-being of the child. Furthermore, we have observed large individual differences in terms of time that is needed for any positive effects of the diet to surface during the elimination phase. Some children appear to be ‘fast-responders’, who show a positive reaction after a few days, whereas other children are ‘slow-responders’, who show a positive reaction after a couple of weeks. It remains to be investigated what could explain these individual differences and whether these individual differences are useful indicators for optimizing the individual treatment by reducing the duration of the treatment. Similarly, individual variation during the reintroduction phase is evident, with aggravation of ADHD symptoms in some children after food reintroduction after 5 days, whereas others only respond after 10–14 days. In addition, children with little variation in their normal food habits tend to respond more strongly to the intervention, but also seem to have the most difficulty in adhering to the diet since they dislike many foods that are present in the elimination phase. This may result in the child eating too few different foods during this phase, increasing the risk for non-adherence since the elimination phase is then overly strict. For the feasibility of this intensive treatment, it is also crucial to consider the optimal moment to start the diet. Stressors or life events, such as change of school environment or family structure, illnesses, but also positive events, such as celebrations and holidays, may negatively impact the motivation and feasibility of the successful application of the treatment. The reintroduction phase is the most intensive part of the treatment given its long duration. Close supervision by professionals when applying this diet or any other elimination diet is necessary such that effects of the diet as well as parent–child relationships can be monitored, the diet can be adjusted if needed, and training, support and guidance can be given to prevent the risk of adverse consequences. Apart from stigmatization, diversion of treatment resources, and nutritional deficiency, parent–child interaction problems may also be a risk. For instance, it has occurred that the child seized the opportunity to put pressure on parents to get what he/she desires with the ‘threat’ of not adhering to the diet. Practitioners should inform the caregivers that an elimination diet requires a change of lifestyle, and success will depend largely on the level of control over the child’s diet and behavior, and the resources and organizational capacities within the family. Finally, caregivers should be reminded that the decision to explore the effects of an elimination diet should be weighed against potential adverse consequences.
Future research is necessary to establish the clinical utility of elimination diets in the treatment of children with ADHD and ASD, particularly considering the widespread use of these treatments. Adequately powered, long-term RCTs, including blinded assessment of the primary outcome, should be conducted to determine the effectiveness and cost-effectiveness of elimination diets for children with ADHD and ASD. In these trials, a reintroduction or food challenge phase, preferably double-blind placebo-controlled, should take place after the elimination phase to determine the effects of the eliminated food items thoroughly. It remains to be investigated whether effects of specific foods can be specific for certain disorders or behavioral problems. In addition, many questions pertaining to the feasibility of these treatments remain to be answered. Attrition rates ranging from 0 to 25% have been reported in the literature, but these numbers were mostly based on a range of short-term trials with large heterogeneity in the designs (e.g., in preselection of patients, duration of trial, and nature of controlled challenge phase of the experiment). Thus, long-term clinical trials are particularly important for answering questions related to feasibility. Finally, this work should investigate how biopsychosocial variables may be affected by the treatment and may play a role in the efficacy of the treatment.
New avenues should also include the identification of predictors of treatment response, especially given that elimination diets may only improve the behavior in a (small) subset of the affected children. Future research might also target the microbiome–gut–brain connection as a potential mechanism that underlies the development of neurodevelopment disorders and elimination diets’ effect in reducing symptoms [126, 136]. A multidisciplinary approach is essential for the investigation of the relation between the microbiome–gut–brain connection and behavioral symptoms of ASD and ADHD, which potentially involves multiple systems, including the metabolic, immune, endocrine, and neural system. The knowledge acquisition pertaining to the mechanism of action could ultimately help inform clinicians about potential markers and targets for preventative and individualized treatment.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington, VA
Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. Lancet 387:1240–1250. doi:10.1016/S0140-6736(15)00238-X
Frazier JA, Biederman J, Bellordre CA et al (2001) Should the diagnosis of attention-deficit/hyperactivity disorder be considered in children with pervasive developmental disorder? J Atten Disord 4:203–211. doi:10.1177/108705470100400402
Holtmann M, Bölte S, Poustka F (2007) Attention deficit hyperactivity disorder symptoms in pervasive developmental disorders: association with autistic behavior domains and coexisting psychopathology. Psychopathology 40:172–177
Hattori J, Ogino T, Abiru K et al (2016) Are pervasive developmental disorders and attention-deficit/hyperactivity disorder distinct disorders? Brain Dev 28:371–374. doi:10.1016/j.braindev.2005.11.009
Simonoff E, Pickles A, Charman T et al (2016) Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 47:921–929. doi:10.1097/CHI.0b013e318179964f
Pelsser LM, Frankena K, Buitelaar JK, Rommelse NN (2010) Effects of food on physical and sleep complaints in children with ADHD: a randomised controlled pilot study. Eur J Pediatr 169:1129–1138. doi:10.1007/s00431-010-1196-5
Rommelse NNJ, Franke B, Geurts HM et al (2010) Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 19:281–295. doi:10.1007/s00787-010-0092-x
Lichtenstein P, Carlström E, Rastam M et al (2010) The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry 167:1357–1363
Mandy W, Lai M-C (2016) Annual Research Review: the role of the environment in the developmental psychopathology of autism spectrum condition. J Child Psychol Psychiatry 57:271–292. doi:10.1111/jcpp.12501
Sandin S, Lichtenstein P, Larsson H et al (2014) The familial risk of autism. JAMA 311:1770–1777. doi:10.1001/jama.2014.4144.The
Bale TL, Baram TZ, Brown AS et al (2010) Early life programming and neurodevelopmental disorders. Biol Psychiatry 68:314–319. doi:10.1016/j.biopsych.2010.05.028.Early
Hoˆtel-Dieu, Notre-Dame P du P (2004) Effects of diet on behaviour and cognition in children France Bellisle. Br J Nutr 92:227. doi:10.1079/BJN20041171
Dauncey MJ (2009) New insights into nutrition and cognitive neuroscience. Proc Nutr Soc 68:408–415. doi:10.1017/S0029665109990188
Goyal MS, Venkatesh S, Milbrandt J et al (2015) Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci 112:14105–14112. doi:10.1073/pnas.1511465112
Graham J, Banaschewski T, Buitelaar J et al (2011) European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry 20:17–37. doi:10.1007/s00787-010-0140-6
Stevenson J, Buitelaar J, Cortese S et al (2014) Research review: the role of diet in the treatment of attention-deficit/hyperactivity disorder—an appraisal of the evidence on efficacy and recommendations on the design of future studies. J Child Psychol Psychiatry Allied Discip 5:416–427. doi:10.1111/jcpp.12215
Sonuga-Barke EJS, Brandeis D, Cortese S et al (2013) Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 170:275–289. doi:10.1176/appi.ajp.2012.12070991
Elder J, Kreider C, Schaefer N, DeLaosa M (2015) A review of gluten- and casein-free diets for treatment of autism: 2005–2015. Nutr Diet Suppl 2015(7):87. doi:10.2147/NDS.S74718
Bozzatello P, Brignolo E, De Grandi E, Bellino S (2016) Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med 5:67. doi:10.3390/jcm5080067
Rowe AH (1944) Elimination diets and the patient’s allergies; a handbook of allergy. Lea & Febiger, Philadelphia
Singh MM, Kay (1976) Wheat gluten as a pathogenic factor in schizophrenia. Science 191:401–402. doi:10.1126/science.1246624
Prugh DG (1951) A preliminary report on the role of emotional factors in idiopathic celiac disease. Psychosom Med 13:220–241
Buie T (2013) The relationship of autism and gluten. Clin Ther 35:578–583. doi:10.1016/j.clinthera.2013.04.011
Coury DL, Ashwood P, Fasano A et al (2012) Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics 130(Suppl):S160–S168. doi:10.1542/peds.2012-0900N
McElhanon BO, McCracken C, Karpen S, Sharp WG (2014) Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133:872–883. doi:10.1542/peds.2013-3995
de Magistris L, Familiari V, Pascotto A et al (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51:418–424. doi:10.1097/MPG.0b013e3181dcc4a5
D’Eufemia P, Celli M, Finocchiaro R et al (1996) Abnormal intestinal permeability in children with autism. Acta Paediatr 85:1076–1079. doi:10.1111/j.1651-2227.1996.tb14220.x
Horvath K, Perman J (2002) Autistic disorder and gastrointestinal disease. Curr Opin Pediatr 14:583–587. doi:10.1097/01.MOP.0000030221.71203.46
Shattock P, Kennedy A, Rowell F, Berney T (1990) Role of neuropeptides in autism and their relationships with classical neurotransmitters. Brain Dysfunct 3:328–345
Panksepp J (1979) A neurochemical theory of autism. Trends Neurosci 2:174–177. doi:10.1016/0166-2236(79)90071-7
Reichelt KL, Ekrem J, Scott H (1990) Gluten, milk proteins and autism: dietary intervention effects on behavior and peptide secretion. J Appl Nutr 42:1–11
Feingold BF (1975) Hyperkinesis and learning disabilities linked to artificial food flavors and colors. Am J Nurs 75:797–803. doi:10.1177/002221947600900902
Pelsser LM, Frankena K, Toorman J et al (2011) Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): a randomised controlled trial. Lancet 377:494–503. doi:10.1016/S0140-6736(10)62227-1
Christison GW, Ivany K (2006) Elimination diets in autism spectrum disorders: any wheat amidst the chaff? J Dev Behav Pediatr 27:162–171
Millward C, Ferriter M, Calver S, Connell-Jones G (2008) Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev CD003498:1–29. doi:10.1002/14651858.CD003498.pub3
Mulloy A, Lang R, O’Reilly M et al (2010) Gluten-free and casein-free diets in the treatment of autism spectrum disorders: a systematic review. Res Autism Spectr Disord 4:328–339. doi:10.1016/j.rasd.2009.10.008
Hurwitz S (2013) The gluten-free, casein-free diet and autism: limited return on family investment. J Early Interv 35:3–19. doi:10.1177/1053815113484807
Whiteley P, Shattock P, Knivsberg A-M et al (2012) Gluten- and casein-free dietary intervention for autism spectrum conditions. Front Hum Neurosci 6:344. doi:10.3389/fnhum.2012.00344
Mari-Bauset S, Zazpe I, Mari-Sanchis A et al (2014) Evidence of the gluten-free and casein-free diet in autism spectrum disorders: a systematic review. J Child Neurol 29:1718–1727. doi:10.1177/0883073814531330
Hyman SL, Stewart PA, Foley J et al (2016) The gluten-free/casein-free diet: a double-blind challenge trial in children with autism. J Autism Dev Disord 46:205–220. doi:10.1007/s10803-015-2564-9
Johnson CR, Handen BL, Zimmer M et al (2011) Effects of gluten free/casein free diet in young children with autism: a pilot study. J Dev Phys Disabil 23:213–225. doi:10.1007/s10882-010-9217-x
Elder JH, Shankar M, Shuster J et al (2006) The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 36:413–420. doi:10.1007/s10803-006-0079-0
Knivsberg AM, Reichelt KL, HØien T, NØdland M (2002) A randomised, controlled study of dietary intervention in autistic syndromes. Nutr Neurosci 5:251–261. doi:10.1080/10284150290028945
Whiteley P, Haracopos D, Knivsberg A-M et al (2010) The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci 13:87–100. doi:10.1179/147683010X12611460763922
Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A (2016) Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr 12:436–442. doi:10.1007/s12519-016-0040-z
Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B (2005) Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology 51:77–85
Kanarek RB (2011) Artificial food dyes and attention deficit hyperactivity disorder. Nutr Rev 69:385–391. doi:10.1111/j.1753-4887.2011.00385.x
Harley JP, Ray RS, Tomasi L et al (1978) Hyperkinesis and food additives: testing the Feingold hypothesis. Pediatrics 61:818–828
Conners CK, Goyette CH, Southwick DA et al (1976) Food additives and hyperkinesis: a controlled double-blind experiment. Pediatrics 58:154–166
Nigg JT, Lewis K, Edinger T, Falk M (2012) Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry 51:86–97. doi:10.1016/j.jaac.2011.10.015
Pelsser LMJ, Buitelaar JK, Savelkoul HFJ (2009) ADHD as a (non) allergic hypersensitivity disorder: a hypothesis. Pediatr Allergy Immunol 20:107–112. doi:10.1111/j.1399-3038.2008.00749.x
Lucarelli S, Frediani T, Zingoni A et al (1995) Food allergy and infantile autism. Panminerva Med 37:137–141
Jyonouchi H, Geng L, Ruby A et al (2016) Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr 146:605–610. doi:10.1016/j.jpeds.2005.01.027
Ashwood P, Enstrom A, Krakowiak P et al (2009) Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol 204:149–153. doi:10.1016/j.jneuroim.2008.07.006.DECREASED
Molloy CA et al (2006) Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol 172:198–205
Ashwood P et al (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Estes ML, McAllister AK (2015) Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neursci 16:469–486
Momemi N, Brudin L, Behnia F, Nordstrom B et al (2012) High complement factor I activity in the plasma of children with autism spectrum disorder. Autism Res Treat 2012:6
De Theije CGM, Bavelaar BM, Lopes da Silva S et al (2014) Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr Allergy Immunol 25:218–226. doi:10.1111/pai.12149
Hak E, de Vries TW, Hoekstra PJ, Jick SS (2016) Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the general practice research database. Ann Allergy Asthma Immunol 111(102–106):e2. doi:10.1016/j.anai.2013.05.023
Suwan P, Akaramethathip D, Noipayak P (2011) Association between allergic sensitization and attention deficit hyperactivity disorder (ADHD). Asian Pac J Allergy Immunol 29:57–65
Egger J, Graham PJ, Carter CM et al (2016) Controlled trial of oligoantigenic treatment in the hyperkinetic syndrome. Lancet 325:540–545. doi:10.1016/S0140-6736(85)91206-1
Verlaet AAJ, Noriega DB, Hermans N, Savelkoul HFJ (2014) Nutrition, immunological mechanisms and dietary immunomodulation in ADHD. Eur Child Adolesc Psychiatry 23:519–529. doi:10.1007/s00787-014-0522-2
Yatsunenko T, Rey FE, Manary MJ et al (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227
Sonnenburg JL, Xu J, Leip DD, et al (2005) Glycan Foraging in Vivo by an Intestine-Adapted Bacterial Symbiont. Science (80-) 307:1955 LP-1959
Olszak T, An D, Zeissig S, et al (2012) Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science (80-) 336:489 LP-493
Candela M, Perna F, Carnevali P et al (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 125:286–292. doi:10.1016/j.ijfoodmicro.2008.04.012
Fukuda S, Toh H, Hase K et al (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547
Dicksved J, Halfvarson J, Rosenquist M et al (2008) Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J 2:716–727
Frank DN, St. Amand AL, Feldman RA et al (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104:13780–13785. doi:10.1073/pnas.0706625104
Lupton JR (2004) Diet induced changes in the colonic environment and colorectal cancer. J Nutr 134:479–482
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Micro 12:661–672
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI (2016) Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3:213–223. doi:10.1016/j.chom.2008.02.015
Zhang C, Zhang M, Wang S et al (2009) Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4:232–241
Gonzalez A, Stombaugh J, Lozupone C et al (2011) The mind-body-microbial continuum. Dialogues Clin Neurosci 13:55–62
Vrieze A, de Groot PF, Kootte RS et al (2016) Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol 27:127–137. doi:10.1016/j.bpg.2013.03.003
Mayer EA (2011) Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12:453–466. doi:10.1038/nrn3071
Neufeld KM, Kang N, Bienenstock J, Foster JA (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23:255–265. doi:10.1111/j.1365-2982.2010.01620.x
Neufeld K-AM, Kang N, Bienenstock J, Foster JA (2011) Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 4:492–494. doi:10.4161/cib.4.4.15702
Bercik P, Denou E, Collins J et al (2016) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141(599–609):e3. doi:10.1053/j.gastro.2011.04.052
Sharma S, Fernandes MF, Fulton S (2013) Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes 37:1183–1191
Li W, Dowd SE, Scurlock B et al (2009) Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav 96:557–567. doi:10.1016/j.physbeh.2008.12.004
Bruce-Keller AJ, Salbaum JM, Luo M et al (2015) Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77:607–615. doi:10.1016/j.biopsych.2014.07.012
Borre YE, O’Keeffe GW, Clarke G et al (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518. doi:10.1016/j.molmed.2014.05.002
Heijtz RD, Wang S, Anuar F et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108:3047–3052. doi:10.1073/pnas.1010529108
Rogers GB, Keating DJ, Young RL et al (2016) From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21:1–11. doi:10.1038/mp.2016.50
Forsyth P, Bienenstock J, Kunze WA (2014) Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817:115–133. doi:10.1007/978-1-4939-0897-4_5
Bravo JA, Forsythe P, Chew MV et al (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 108:16050–16055. doi:10.1073/pnas.1102999108
Cryan JF, Kaupmann K (2005) Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43
Severance EG, Gressitt KL, Stallings CR et al (2016) Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 148:130–137. doi:10.1016/j.schres.2013.05.018
Bailey MT, Dowd SE, Galley JD et al (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25:397–407. doi:10.1016/j.bbi.2010.10.023
Furusawa Y, Obata Y, Fukuda S et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–450
Smith PM, Howitt MR, Panikov N et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. doi:10.1126/science.1241165
Donohoe DR, Garge N, Zhang X et al (2016) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13:517–526. doi:10.1016/j.cmet.2011.02.018
MacFabe DF (2015) Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb Ecol Health Dis 26. doi:10.3402/mehd.v26.28177. doi:10.3402/mehd.v26.28177
Kimura I, Inoue D, Maeda T et al (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci 108:8030–8035. doi:10.1073/pnas.1016088108
Nøhr MK, Pedersen MH, Gille A et al (2013) GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154:3552–3564. doi:10.1210/en.2013-1142
Desbonnet L, Garrett L, Clarke G et al (2016) The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43:164–174. doi:10.1016/j.jpsychires.2008.03.009
O’Mahony SM, Clarke G, Borre YE et al (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48. doi:10.1016/j.bbr.2014.07.027
Yano JM, Donaldson GP, Shastri GG, Ann P et al (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276. doi:10.1016/j.cell.2015.02.047
Tsavkelova EA, Botvinko IV, Kudrin VS, Oleskin AV (2000) Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem 372:115–117
Barrett E, Ross RP, O’Toole PW et al (2012) γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113:411–417. doi:10.1111/j.1365-2672.2012.05344.x
Thomas CM, Hong T, van Pijkeren JP et al (2012) Histamine derived from probiotic Lactobacillus Reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 7:e31951
Stephenson M, Rowatt E, Harrison K (1947) The production of acetylcholine by a strain of Lactobacillus plantarum. Microbiology 1:279–298
Grenham S, Clarke G, Cryan J, Dinan T (2011) Brain–gut–microbe communication in health and disease. Front Physiol 2:94
Palmer C, Bik EM, DiGiulio DB et al (2007) Development of the human infant intestinal microbiota. PLoS Biol 5:e177
Mackie RI, Sghir A, Gaskins HR (1999) Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 69:1035s–1045s
Sudo N, Chida Y, Aiba Y et al (2004) Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 5581:263–275. doi:10.1113/jphysiol.2004.063388
Adams JB, Johansen LJ, Powell LD et al (2011) Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11:22. doi:10.1186/1471-230X-11-22
Finegold SM, Molitoris D, Song Y et al (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35:S6–S16. doi:10.1086/341914
Song Y, Liu C, Finegold SM (2004) Real-time PCR quantitation of clostridia in feces of autistic children real-time PCR quantitation of clostridia in feces of autistic children. Appl Enviromental Microbiol 70:6459–6465. doi:10.1128/AEM.70.11.6459
Kang DW, Park JG, Ilhan ZE et al (2013) Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. doi:10.1371/journal.pone.0068322
Williams BL, Hornig M, Buie T et al (2011) Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. doi:10.1371/journal.pone.0024585
Finegold SM, Dowd SE, Gontcharova V et al (2010) Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16:444–453. doi:10.1016/j.anaerobe.2010.06.008
Parracho HMRT, Bingham MO, Gibson GR, McCartney AL (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54:987–991. doi:10.1099/jmm.0.46101-0
Curran LK, Newschaffer CJ, Lee L-C, et al (2007) Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120:e1386 LP-e1392
Sandler RH, Finegold SM, Bolte ER et al (2000) Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 15:429–435. doi:10.1177/088307380001500701
Critchfield JW, Van Hemert S, Ash M et al (2011) The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. doi:10.1155/2011/161358
Hsiao EY, McBride SW, Hsien S et al (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. doi:10.1016/j.cell.2013.11.024
Cass H, Gringras P, March J et al (2008) Absence of urinary opioid peptides in children with autism. Arch Dis Child. doi:10.1136/adc.2006.114389
Heberling CA, Dhurjati PS, Sasser M (2016) Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses 80:264–270. doi:10.1016/j.mehy.2012.11.044
Maes M (2007) Leaky gut in chronic fatigue syndrome: a review. Activ Nerv Super 51:21–28
Maes M, Coucke F, Leunis JC (2007) Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro Endocrinol Lett 28:739–744
Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518. doi:10.1016/j.molmed.2014.05.002
Scheres A, Milham MP, Knutson B, Castellanos FX (2016) Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 61:720–724. doi:10.1016/j.biopsych.2006.04.042
Ströhle A, Stoy M, Wrase J et al (2008) Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39:966–972. doi:10.1016/j.neuroimage.2007.09.044
Maljaars J, Boonen H, Lambrechts G et al (2014) Maternal parenting behavior and child behavior problems in families of children and adolescents with autism spectrum disorder. J Autism Dev Disord 44:501–512. doi:10.1007/s10803-013-1894-8
Pressman LJ, Loo SK, Carpenter EM et al (2016) Relationship of family environment and parental psychiatric diagnosis to impairment in ADHD. J Am Acad Child Adolesc Psychiatry 45:346–354. doi:10.1097/01.chi.0000192248.61271.c8
Foley M (2011) A comparison of family adversity and family dysfunction in families of children with attention deficit hyperactivity disorder (ADHD) and families of children without ADHD. J Spec Pediatr Nurs 16:39–49. doi:10.1111/j.1744-6155.2010.00269.x
Wyatt Kaminski J, Valle LA, Filene JH, Boyle CL (2008) A meta-analytic review of components associated with parent training program effectiveness. J Abnorm Child Psychol 36:567–589. doi:10.1007/s10802-007-9201-9
Daley D, van der Oord S, Ferrin M et al (2016) Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 53(835–847):e5. doi:10.1016/j.jaac.2014.05.013
Pelsser LM, van Steijn DJ, Frankena K et al (2013) A randomized controlled pilot study into the effects of a restricted elimination diet on family structure in families with ADHD and ODD. Child Adolesc Ment Health 18:39–45. doi:10.1111/j.1475-3588.2012.00652.x
Hediger ML, England LJ, Molloy CA et al (2008) Reduced bone cortical thickness in boys with autism or autism spectrum disorder. J Autism Dev Disord 38:848–856. doi:10.1007/s10803-007-0453-6
Petra AI, Panagiotidou S, Hatziagelaki E et al (2016) Gut-microbiota-brain axis and effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37:984–995. doi:10.1016/j.clinthera.2015.04.002.Gut-microbiota-brain
Acknowledgements
This work was supported by Grants by ZonMw, doelmatigheidsonderzoek Programme (837002506) and by Innovatiefonds Zorgverzekeraars, and by the Horizon2020 Programme Eat2BeNICE (728018), awarded to NR and JB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
This article is part of the Focused Issue "The role of nutrition in child and adolescent onsetmental disorders” of European Child and Adolescent Psychiatry.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ly, V., Bottelier, M., Hoekstra, P.J. et al. Elimination diets’ efficacy and mechanisms in attention deficit hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 26, 1067–1079 (2017). https://doi.org/10.1007/s00787-017-0959-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-0959-1