Abstract
Objectives
This study compared the chemical composition, microstructural, and mechanical properties of human and bovine dentin subjected to a demineralization/remineralization process.
Materials and methods
Human and bovine incisors were sectioned to obtain 120 coronal dentin beams (6 × 1 × 1 mm3) that were randomly allocated into 4 subgroups (n = 15) according to the time of treatment (sound, pH-cycling for 3, 7, and 14 days). Three-point bending mechanical test, attenuated total reflectance–Fourier transform infrared (ATR-FTIR), thermogravimetric (TG), and X-ray diffraction (XRD) techniques were employed to characterize the dentin samples.
Results
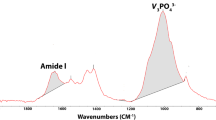
Regarding chemical composition at the molecular level, bovine sound dentin showed significantly lower values in organic and inorganic content (collagen cross-linking, CO3/amide I, and CO3/PO4; p = 0.002, p = 0.026, and p = 0.002, respectively) compared to humans. Employing XRD analyses, a higher mineral crystallinity in human dentin than in bovines at 7 and 14 days (p = 0.003 and p = 0.009, respectively) was observed. At the end of the pH-cycling, CI (ATR-FTIR) and CO3/PO4 ratios (ATR-FTIR) increased, while CO3/amide I (ATR-FTIR), PO4/amide I (ATR-FTIR), and %mineral (TG) ratios decreased. The extension by compression values increased over exposure time with significant differences between dentin types (p < 0.001, in all cases), reaching higher values in bovine dentin. However, flexural strength (MPa) did not show differences between groups. We also observed the correlation between compositional variables (i.e., PO4/amide I, CI, and %mineral) and the extension by compression.
Conclusions
Human and bovine dentin are different in terms of microstructure, chemical composition, mechanical strength, and in their response to the demineralization/remineralization process by pH-cycling.
Clinical relevance
These dissimilarities may constitute a potential limitation when replacing human teeth with bovines in in vitro studies.



Similar content being viewed by others
References
Yassen GH, Platt JA, Hara AT (2011) Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci 53:273–282. https://doi.org/10.2334/josnusd.53.273
Soares FZM, Follak A, da Rosa LS, Montagner AF, Lenzi TL, Rocha RO (2016) Bovine tooth is a substitute for human tooth on bond strength studies: a systematic review and meta-analysis of in vitro studies. Dent Mater 32:1385–1393. https://doi.org/10.1016/j.dental.2016.09.019
Nakamichi I, Iwaku M, Fusayama T (1983) Bovine teeth as possible substitutes in the adhesion test. J Dent Res 62:1076–1081. https://doi.org/10.1177/00220345830620101501
Wegehaupt FJ, Widmer R, Attin T (2010) Is bovine dentine an appropriate substitute in abrasion studies? Clin Oral Investig 14:201–205. https://doi.org/10.1007/s00784-009-0283-3
Tanaka JLO, Medici Filho E, Salgado JAP, Salgado MAC, Moraes LC, Moraes MEL, Castilho JCM (2008) Comparative analysis of human and bovine teeth: radiographic density. Braz Oral Res 22:346–351. https://doi.org/10.1590/S1806-83242008000400011
Dutra-Correa M, Anauate-Netto C, Arana-Chavez VE (2007) Density and diameter of dentinal tubules in etched and non-etched bovine dentine examined by scanning electron microscopy. Arch Oral Biol 52:850–855. https://doi.org/10.1016/j.archoralbio.2007.03.003
Lopes MB, Sinhoreti MAC, Gonini Júnior A, Consani S, Mccabe JF (2009) Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz Dent J 20:279–283. https://doi.org/10.1590/S0103-64402009000400003
Fonseca RB, Haiter-Neto F, Fernandes-Neto AJ, Barbosa GAS, Soares CJ (2004) Radiodensity of enamel and dentin of human, bovine and swine teeth. Arch Oral Biol 49:919–922. https://doi.org/10.1016/j.archoralbio.2004.05.006
Marquezan M, Corrêa FNP, Sanabe ME, Rodrigues Filho LE, Hebling J, Guedes-Pinto AC, Mendes FM (2009) Artificial methods of dentine caries induction: a hardness and morphological comparative study. Arch Oral Biol 54:1111–1117. https://doi.org/10.1016/j.archoralbio.2009.09.007
White DJ (1995) The application of in vitro models to research on demineralization and remineralization of the teeth. Adv Dent Res 9:175–193
Pacheco LF, Banzi ÉC d F, Rodrigues E et al (2013) Molecular and structural evaluation of dentin caries-like lesions produced by different artificial models. Braz Dent J 24:610–618. https://doi.org/10.1590/0103-6440201302357
Boskey AL, Mendelsohn R (2005) Infrared spectroscopic characterization of mineralized tissues. Vib Spectrosc 38:107–114. https://doi.org/10.1016/j.vibspec.2005.02.015
Lopes C d CA, Limirio PHJO, Novais VR, Dechichi P (2018) Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl Spectrosc Rev 53:747–769. https://doi.org/10.1080/05704928.2018.1431923
Rodriguez-Navarro A, Romanek C, Alvarez-LLoret P, Gaines K (2006) Effect of in ovo exposure to PCBs and Hg on clapper rail bone mineral chemistry from a contaminated salt marsh in coastal Georgia. Environ Sci Technol 40:4936–4942. https://doi.org/10.1021/es060769x
Miller LM, Vairavamurthy V, Chance MR, Mendelsohn R, Paschalis EP, Betts F, Boskey AL (2001) In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the ν4 PO43− vibration. Biochim Biophys Acta, Gen Subj 1527:11–19. https://doi.org/10.1016/S0304-4165(01)00093-9
Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M (2001) Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res 16:1821–1828. https://doi.org/10.1359/jbmr.2001.16.10.1821
Holager J (1970) Thermogravimetric examination of enamel and dentin. J Dent Res 49:546–548
Lim JJ, Liboff AR (1972) Thermogravimetric analysis of dentin. J Dent Res 51:509–514. https://doi.org/10.1177/00220345720510024401
Elfersi S, Grégoire G, Sharrock P (2002) Characterization of sound human dentin particles of sub-millimeter size. Dent Mater 18:529–534. https://doi.org/10.1586/ecp.10.28
Reyes-Gasga J, Martínez-Piñeiro EL, Rodríguez-Álvarez G, Tiznado-Orozco GE, García-García R, Brès EF (2013) XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater Sci Eng C 33:4568–4574. https://doi.org/10.1016/j.msec.2013.07.014
Gadaleta SJ, Paschalis EP, Betts F, Mendelsohn R, Boskey AL (1996) Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between X-ray diffraction and infrared data. Calcif Tissue Int 58:9–16. https://doi.org/10.1007/BF02509540
Hara AT, Queiroz CS, Paes Leme AF, Serra MC, Cury JA (2003) Caries progression and inhibition in human and bovine root dentine in situ. Caries Res 37:339–344. https://doi.org/10.1159/000072165
De Dios TJ, Alcolea A, Hernández A, Ruiz AJO (2015) Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol 60:768–775. https://doi.org/10.1016/j.archoralbio.2015.01.014
Silva Soares LE, Do Espírito Santo AM (2015) Morphological and chemical comparative analysis of the human and bovine dentin-adhesive layer. Microsc Microanal 21:204–213. https://doi.org/10.1017/S143192761401366X
Falla-Sotelo FO, Rizzutto MA, Tabacniks MH, Added N, Barbosa MDL, Markarian RA, Quinelato A, Mori M, Youssef M (2005) Analysis and discussion of trace elements in teeth of different animal species. Braz J Phys 35:761–762. https://doi.org/10.1590/S0103-97332005000500010
Soares LES, Campos ADF, Martin AA (2013) Human and bovine dentin composition and its hybridization mechanism assessed by FT-Raman spectroscopy. J Spectrosc 2013:1–7. https://doi.org/10.1155/2013/210671
Dawes C (2003) What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc 69:722–725
Ito A, Maekawa K, Tsutsumi S, Ikazaki F, Tateishi T (1997) Solubility product of OH-carbonated hydroxyapatite. J Biomed Mater Res 36:522–528. https://doi.org/10.1002/(SICI)1097-4636(19970915)36:4<522::AID-JBM10>3.0.CO;2-C
Ortiz-Ruiz AJ, Teruel-Fernández J d D, Alcolea-Rubio LA et al (2018) Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Ann Anat 218:7–17. https://doi.org/10.1016/j.aanat.2017.12.012
Barralet JE, Best S, Bonfield W (1998) Carbonate substitution in precipitated hydroxyapaptite: an investigation into the effects of reaction temperature and bicarbonae ion concentration. J Biomed Mater Res 41:79–86
Rey C, Collins B, Goehl T, Dickson IR, Glimcher MJ (1989) The carbonate environment in bone mineral: a resolution-enhanced fourier transform infrared spectroscopy study. Calcif Tissue Int 45:157–164. https://doi.org/10.1007/BF02556059
Donnelly E, Boskey AL, Baker SP, van der Meulen MCH (2010) Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A 92:1048–1056. https://doi.org/10.1002/jbm.a.32442
Kinney J, Balooch M, Haupt D et al (1995) Mineral distribuition and dimensional changes in human dentin during demineralization. J Dent Res 74:1179–1184
Almahdy A, Downey FC, Sauro S, Cook RJ, Sherriff M, Richards D, Watson TF, Banerjee A, Festy F (2012) Microbiochemical analysis of carious dentine using raman and fluorescence spectroscopy. Caries Res 46:432–440. https://doi.org/10.1159/000339487
Cazalbou S, Combes C, Eichert D et al (2004) Poorly crystalline apatites: evolution and maturation in vitro and in vivo. J Bone Miner Metab 22:310–317. https://doi.org/10.1007/s00774-004-0488-0
Dominguez-Gasca N, Benavides-Reyes C, Sánchez-Rodríguez E, Rodríguez-Navarro AB (2019) Changes in avian cortical and medullary bone mineral composition and organization during acid-induced demineralization. Eur J Mineral 31:209–216. https://doi.org/10.1127/ejm/2019/0031-2826
Ryou H, Amin N, Ross A, Eidelman N, Wang DH, Romberg E, Arola D (2011) Contributions of microstructure and chemical composition to the mechanical properties of dentin. J Mater Sci Mater Med 22:1127–1135. https://doi.org/10.1007/s10856-011-4293-8
Schilke R, Lisson JA, Bauß O, Geurtsen W (2000) Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol 45:355–361. https://doi.org/10.1016/S0003-9969(00)00006-6
Camargo CHR, Siviero M, Camargo SEA, de Oliveira SHG, Carvalho CAT, Valera MC (2007) Topographical, diametral, and quantitative analysis of dentin tubules in the root canals of human and bovine teeth. J Endod 33:422–426. https://doi.org/10.1016/J.JOEN.2006.12.011
Ferreira M, De Carvalho F, Neiva C (2018) Viability of bovine teeth as a substrate in bond strength tests : a systematic review and meta-analysis. J Adhes Dent 20:471–480. https://doi.org/10.3290/j.jad.a41636
Acknowledgments
This work was supported by Research Projects of the Spanish government [grant number CGL2015-64683-P]. We thank Dr. A.F.M.A. Chowdhruy for his helpful suggestions and remarks.
Funding
The work was supported by Research Projects of the Spanish Government [grant number CGL2015-64683-P].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The conceptualization, methodology, formal analysis, and original draft were performed by Tattiana Enrich-Essvein, Cristina Benavides-Reyes, María Victoria Bolaños-Carmona, and Santiago González-López. The supervision, validation, writing, review, and editing were made by Pedro Álvarez-Lloret and Alejandro B Rodríguez-Navarro. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Declarations
Approval was obtained from the ethics committee of University of Granada, Spain (#1006-2019). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Enrich-Essvein, T., Benavides-Reyes, C., Álvarez-Lloret, P. et al. Influence of de-remineralization process on chemical, microstructural, and mechanical properties of human and bovine dentin. Clin Oral Invest 25, 841–849 (2021). https://doi.org/10.1007/s00784-020-03371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03371-9