Abstract
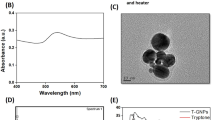
Gold nanoparticles (GNPs) of different sizes and shapes have been investigated extensively for their therapeutic potential against several diseases including cancer. However, the mechanisms with which they affect the cells are yet to be fully comprehended. In this study, we report the strong antiproliferative potential of novel, star-shaped (“stellate”) GNPs that target tubulin—the building-block protein of the cytoskeletal filaments called microtubules—and disrupt microtubule network integrity. The stellate GNPs (“sGNPs”) were synthesized from tryptone-stabilized GNPs (“tGNPs”) and characterized by various spectroscopy methods combined with high-resolution transmission electron microscopy. Among a panel of cancer cell lines tested, they showed strong antiproliferative and anti-clonogenic efficacy against MDA-MB-231 cells. The antiproliferative mechanism of the sGNPs involves perturbation of the secondary and tertiary conformation of tubulin as evidenced by far-UV circular dichroism and anilinonaphthalene sulphate-binding assays. The structural perturbation of tubulin retarded its assembly competence as evidenced by polymer mass analysis and electron microscopy imaging of tubulin assembled in vitro and by immunofluorescence visualization of the cellular microtubules. The treated cells also induced cell cycle arrest at G1 phase. Taken together, our data suggest that sGNPs are potent, tubulin-targeted antiproliferative particles that can be evaluated further for their anticancer potential.




Similar content being viewed by others
References
Faraday M (1857) The Bakerian lecture: experimental relations of gold (and other metals) to light. Philos Trans R Soc Lond. https://doi.org/10.1098/rstl.1857.0011
Woźniak A, Malankowska A, Nowaczyk G et al (2017) Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J Mater Sci Mater Med 28:92. https://doi.org/10.1007/s10856-017-5902-y
Ou YC, Webb JA, O’Brien CM et al (2018) Diagnosis of immunomarkers: in vivo via multiplexed surface-enhanced Raman spectroscopy with gold nanostars. Nanoscale 10:13092–13105. https://doi.org/10.1039/c8nr01478g
Golchin K, Golchin J, Ghaderi S et al (2018) Gold nanoparticles applications: from artificial enzyme till drug delivery. Artif Cells Nanomed Biotechnol 46:250–254. https://doi.org/10.1080/21691401.2017.1305393
García Calavia P, Bruce G, Pérez-García L, Russell DA (2018) Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem Photobiol Sci 17:1534–1552. https://doi.org/10.1039/C8PP00271A
Pedrosa P, Mendes R, Cabral R et al (2018) Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci Rep 8:11429. https://doi.org/10.1038/s41598-018-29870-0
Chen H, Zhang X, Dai S et al (2013) Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 3:633–649. https://doi.org/10.7150/thno.6630
Yuan H, Fales AM, Vo-Dinh T (2012) TAT peptide-functionalized gold nanostars: enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J Am Chem Soc 134:11358–11361. https://doi.org/10.1021/ja304180y
Lopus M (2017) Editorial:tubulin-targeted cancer chemotherapeutics: advances and challenges. Curr Top Med Chem 17:2522. https://doi.org/10.2174/156802661722170726113614
Mahaddalkar T, Lopus M (2017) From natural products to designer drugs: development and molecular mechanisms action of novel anti-microtubule breast cancer therapeutics. Curr Top Med Chem 17:2559. https://doi.org/10.2174/1568026617666170104144240
Libutti SK, Paciotti GF, Myer L et al (2009) Results of a completed phase I clinical trial of CYT-6091: a pegylated colloidal gold-TNF nanomedicine. J Clin Oncol 27:3586. https://doi.org/10.1200/jco.2009.27.15s.3586
Identifier: NCT00848042 (2016) Pilot study of AuroLase (tm) therapy in refractory and/or recurrent tumors of the head and neck. https://www.clinicaltrials.gov
Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV–Vis spectra. Anal Chem 79:4215. https://doi.org/10.1021/ac0702084
Sujitha MV, Kannan S (2013) Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim Acta Part A Mol Biomol Spectrosc 102:15. https://doi.org/10.1016/j.saa.2012.09.042
Suman TY, Radhika Rajasree SR, Ramkumar R et al (2014) The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim Acta Part A Mol Biomol Spectrosc 118:11. https://doi.org/10.1016/j.saa.2013.08.066
Pradhan M, Suri C, Choudhary S et al (2018) Elucidation of the anticancer potential and tubulin isotype-specific interactions of β-sitosterol. J Biomol Struct Dyn 36:195. https://doi.org/10.1080/07391102.2016.1271749
Buch K, Peters T, Nawroth T et al (2012) Determination of cell survival after irradiation via clonogenic assay versus multiple MTT assay—a comparative study. Radiat Oncol 7:1–6. https://doi.org/10.1186/1748-717X-7-1
Cheriyamundath S, Mahaddalkar T, Save SN et al (2018) Aqueous extract of Triphala inhibits cancer cell proliferation through perturbation of microtubule assembly dynamics. Biomed Pharmacother 98:76–81. https://doi.org/10.1016/j.biopha.2017.12.022
Mahaddalkar T, Naik PK, Choudhary S et al (2017) Structural investigations into the binding mode of a novel noscapine analogue, 9-(4-vinylphenyl) noscapine, with tubulin by biochemical analyses and molecular dynamic simulations. J Biomol Struct Dyn 35:2475–2484. https://doi.org/10.1080/07391102.2016.1222969
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Srivastava S, Mishra S, Surolia A, Panda D (2016) C1, a highly potent novel curcumin derivative, binds to tubulin, disrupts microtubule network and induces apoptosis. Biosci Rep 36:e00323–e00323. https://doi.org/10.1042/bsr20160039
Cheriyamundath S, Choudhary S, Lopus M (2018) Safranal Inhibits HeLa cell viability by perturbing the reassembly potential of microtubules. Phyther Res 32:170–173. https://doi.org/10.1002/ptr.5938
Mahaddalkar T, Mehta S, Cheriyamundath S et al (2017) Tryptone-stabilized gold nanoparticles target tubulin and inhibit cell viability by inducing an unusual form of cell cycle arrest. Exp Cell Res 360:163–170. https://doi.org/10.1016/j.yexcr.2017.09.002
Liu Y, Liu L, Yuan M, Guo R (2013) Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloids Surf A Physicochem Eng Asp 417:18–25. https://doi.org/10.1016/j.colsurfa.2012.08.050
Selvakannan PR, Mandal S, Phadtare S et al (2003) Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir 19:3545–3549. https://doi.org/10.1021/la026906v
Verma MS, Chen PZ, Jones L, Gu FX (2014) Branching and size of CTAB-coated gold nanostars control the colorimetric detection of bacteria. RSC Adv 4:10660. https://doi.org/10.1039/c3ra46194g
Viet Long N, Ohtaki M, Yuasa M et al (2013) Synthesis and self-assembly of gold nanoparticles by chemically modified polyol methods under experimental control. J Nanomater 2013:8. https://doi.org/10.1155/2013/793125
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662. https://doi.org/10.1021/nl052396o
Mu Q, Wang H, Zhang M (2017) Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin Drug Deliv 14:123–136. https://doi.org/10.1080/17425247.2016.1208650
Acknowledgements
The authors thank UM-DAE Centre for Excellence in Basic Sciences for financial support, and Indian Institute of Technology Bombay flow cytometry facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2019_1694_MOESM1_ESM.pdf
Supplementary material 1 (PDF 66 kb) Supplementary Fig. 1. Effect of sGNPs on the intrinsic tryptophan fluorescence of BSA. The particles did not show quenching of intrinsic tryptophan fluorescence of the protein. The graph represents one of two independent experiments
Rights and permissions
About this article
Cite this article
Nirmala, J.G., Beck, A., Mehta, S. et al. Perturbation of tubulin structure by stellate gold nanoparticles retards MDA-MB-231 breast cancer cell viability. J Biol Inorg Chem 24, 999–1007 (2019). https://doi.org/10.1007/s00775-019-01694-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01694-x