Abstract
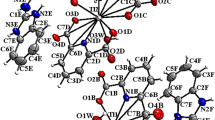
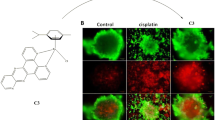
2-Amino-5,10-dihydro-5,10-dioxo-4(pyridine-3-yl)-4H-benzo[g]chromene-3-carbonitrile 5, a cytotoxic lawsone derivative, was reacted with [Ru(p-cymene)Cl2]2 to afford a new Ru(II) ‘piano-stool’ complex 6 which differed from its ligand 5 by a greater selectivity for highly invasive 518A2 melanoma cells over human dermal fibroblasts in MTT cytotoxicity assays, and by inducing senescence rather than apoptosis in the former. DNA is a likely cellular target of complex 6 as it bound, presumably non-covalently, to linear and circular double-stranded DNA in vitro and as ruthenium was found in the lysate of nuclei of treated 518A2 melanoma cells. It also caused a fivefold increase of reactive oxygen species in these cells, originating from a more persistent redox cycling as visualised by cyclic voltammetry.






Similar content being viewed by others
References
Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L et al (2016) Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—update 2016. Eur J Cancer 63:201–217
Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S (2018) Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2:CD011123
Antonarakis ES, Emadi A (2010) Ruthenium-based chemotherapeutics: are they ready for prime time? Cancer Chemother Pharmacol 66:1–9
Rademaker-Lakhai JM, van den Bongard D, Pluim D, Beijnen JH, Schellens JH (2004) A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin Cancer Res 10:3717–3727
Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W et al (2008) KP1019 a new redox-active anticancer agent—preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers 5:2140–2155
Burris HA, Bakewell S, Bendell JC, Infante J, Jones SF, Spigel DR et al (2016) Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 1:e000154
Allardyce CS, Dyson PJ (2001) Ruthenium in medicine: current clinical uses and future prospects. Platin Metals Rev 45:62–69
Biersack B (2016) Anticancer activity and modes of action of (arene) ruthenium(II) complexes coordinated to C-, N-, and O-ligands. Mini Rev Med Chem 16:804–814
Pettinari R, Petrini A, Marchetti F, Pettinari C, Riedel T, Therrien B et al (2017) Arene-ruthenium(II) complexes with bioactive ortho-hydroxydibenzoylmethane ligands: synthesis, structure, and cytotoxicity. Eur J Inorg Chem 12:1800–1806
Schmitt F, Kasparkova J, Brabec V, Begemann G, Schobert R, Biersack B (2018) New (arene)ruthenium(II) complexes of 4-aryl-4H-naphthopyrans with anticancer and anti-vascular activities. J Inorg Biochem 184:69–78
Thota S, Rodrigues DA, Crans DC, Barreiro EJ (2018) Ru(II) compounds: next-generation anticancer metallotherapeutics? J Med Chem 61:5805–5821
Biersack B, Zoldakova M, Effenberger K, Schobert R (2010) (Arene)Ru(II) complexes of epidermal growth factor receptor inhibiting tyrophostins with enhanced selectivity and cytotoxicity in cancer cells. Eur J Med Chem 45:1972–1975
Pradhan R, Dandawate P, Vyas A, Padhye S, Biersack B, Schobert R et al (2012) From body art to anticancer activities: perspectives on medical properties of henna. Curr Drug Target 13:1777–1798
Nadkarni KM (1908) Indian plants and drugs, 1st edn. Nortan & Co., Madras
Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS, Kwon TK (2014) β-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis 5:e1230
Magedov IV, Kireev AS, Jenkins AR, Evdokimov NM, Lima DT, Tongwa P et al (2012) Structural simplification of bioactive natural products with multicomponent synthesis. 4H-pyrano-[2,3-b]naphthoquinones with anticancer activity. Bioorg Med Chem Lett 22:5195–5198
Benimetskaya L, Ayyanar K, Kornblum N, Castanotto D, Rossi J, Wu S et al (2006) Bcl-2 protein in 518A2 melanoma cells in vivo and in vitro. Clin Cancer Res 12:4940–4948
Zerp SF, Van Elsas A, Peltenburg LTC, Schrier PI (1999) p53 mutations in human cutaneous melanoma correlate with sun exposure but are not always involved in melanomagenesis. Br J Cancer 79:921–926
Brabec V, Novakova O (2006) DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug Resist Update 9:111–122
Intini FP, Zajac J, Novohradsky V, Saltarella T, Pacifico C et al (2017) Novel antitumor platinum(II) conjugates containing the nonsteroidal anti-inflammatory agent diclofenac: synthesis and dual mechanism of antiproliferative effects. Inorg Chem 56:1483–1497
Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS et al (2013) Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8:e81162
Kapitza S, Jakupec MA, Uhl M, Keppler BK, Marian B (2005) The heterocyclic ruthenium(III) complex KP1019 (FFC14A) causes DNA damage and oxidative stress in colorectal tumor cells. Cancer 226:115–121
Park EJ, Choi KS, Kwon TK (2011) β-Lapachone-induced reactive oxygen species (ROS) generation mediates autophagic cell death in glioma U87 MG cells. Chem Biol Interact 189:37–44
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Itahana K, Campisi J, Dimri GP (2004) Mechanisms of cellular senescence in human and mouse cells. Biogerontology 5:1–10
Debacq-Chainiaux F, Ameur RB, Bauwens E, Dumortier E, Toutfaire M, Toussaint O (2016) Stress-induced (premature) senescence. In: Rattan S, Hayflick L (eds) Cellular ageing and replicative senescence. Healthy Ageing and longevity, 4th edn. Springer, Cham, pp 243–262
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603
Gire V, Dulic V (2015) Senescence from G2 arrest, revisited. Cell Cycle 14:297–304
Sikora E, Mosieniak G, Sliwinska MA (2016) Morphological and functional characteristics of senescent cells. Curr Drug Targets 17:377–387
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113:3613–3622
Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J (2016) Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—a systematic review of the literature. Clin Epidemiol 8:109–122
Toussaint O, Royer V, Salmon M, Remacle J (2002) Stress-induced premature senescence and tissue ageing. Biochem Pharmacol 64:1007–1009
Berns A (2002) Senescence: a companion in chemotherapy? Cancer Cell 1:309–311
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62:1876–1883
Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y et al (1999) A senescent-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res 59:3761–3767
Roninson IB, Broude EV, Chang BD (2001) If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Update 4:303–313
Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA 92:4337–4341
Ewald JA, Desotelle JA, Wilding G, Jarrard DF (2010) Therapy-induced senescence in cancer. J Natl Cancer Inst 102:1536–1546
Nardella C, Clohessy JG, Alimonti A, Pandolfi PP (2011) Pro-senescence therapy for cancer treatment. Nat Rev Cancer 11:503–511
Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW (2002) A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335–346
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al (2007) Senescence and tumor clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660
Martin L, Schilder RJ (2006) Novel non-cytotoxic therapy in ovarian cancer: current status and future prospects. J Natl Comp Cancer Netw 4:955–966
Winquist E, Waldron T, Berry S, Ernst DS, Hotte S, Lukka H (2006) Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systemic review from the cancer care Ontario program in evidence-based care’s genitourinary cancer disease site group. BMC Cancer 6:112
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Lee M, Lee JS (2014) Exploiting tumor cell senescence in anticancer therapy. BMB Rep 47:51–59
Freund A, Orjalo V, Desprez PY, Campisi J (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16:238–246
Soengas MS, Lowe SW (2003) Apoptosis and melanoma chemoresistance. Oncogene 22:3138–3151
Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA et al (2008) C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene 27:6623–6634
Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA et al (2006) Cellular Senescence in Naevi and immortalization in melanoma: a role for p16? Br J Cancer 95:496–505
Michaloglou C, Vredeveld L, Soengas M, Denoyelle C, Kuilman T, van der Horst C et al (2005) BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436:720–724
Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ, Hersey P (2007) Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer 97:1225–1233
Acknowledgements
R. S. thanks the Deutsche Forschungsgemeinschaft (DFG) for a grant (Scho 402/12-2), H. K., J. K. and V. B. were supported by the Czech Science Foundation (Grant 17–05302S).
Funding
Deutsche Forschungsgemeinschaft Grant Scho 402/12; Czech Science Foundation Grant 17–05302S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2019_1677_MOESM1_ESM.pdf
Stability tests via 1H-NMR for compound 6; electrophoretic mobility shift assays; interaction with pBR322 plasmid DNA; uptake of complex 6 by 518A2 melanoma cells; cyclic voltammetry; caspase-3/7 activity assays; This material is available free of charge via the Internet at https://link.springer.com 1 (PDF 1103 kb)
Rights and permissions
About this article
Cite this article
Gold, M., Mujahid, Y., Ahmed, K. et al. A new 4-(pyridinyl)-4H-benzo[g]chromene-5,10-dione ruthenium(II) complex inducing senescence in 518A2 melanoma cells. J Biol Inorg Chem 24, 647–657 (2019). https://doi.org/10.1007/s00775-019-01677-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01677-y