Abstract
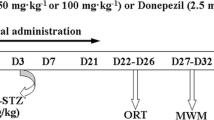
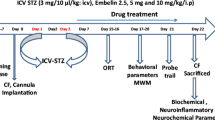
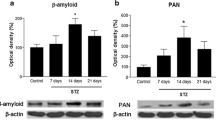
The established decrease in the level of endogenous kyotorphin (KTP) into the cerebrospinal fluid of patients with an advanced stage of Alzheimer’s disease (AD) and the found neuroprotective activity of KTP suggested its participation in the pathophysiology of the disease. We aimed to study the effects of subchronic intracerebroventricular (ICV) treatment (14 days) with KTP on the behavioral, biochemical and histological changes in rats with streptozotocin (STZ-ICV)-induced model of sporadic AD (sAD). Three months after the administration of STZ-ICV, rats developed increased locomotor activity, decreased level of anxiety, impaired spatial and working memory. Histological data from the STZ-ICV group demonstrated decreased number of neurons in the CA1 and CA3 subfields of the hippocampus. The STZ-ICV group was characterized with a decrease of total protein content in the hippocampus and the prefrontal cortex as well as increased levels of the carbonylated proteins in the hippocampus. KTP treatment of STZ-ICV rats normalized anxiety level and regained object recognition memory. KTP abolished the protein loss in prefrontal cortex and decrease the neuronal loss in the CA3 subfield of the hippocampus. STZ-ICV rats, treated with KTP, did not show significant changes in the levels of the carbonylated proteins in specific brain structures or in motor activity and spatial memory compared to the saline-treated STZ-ICV group. Our data show a moderate and selective protective effect of a subchronic ICV administration of the dipeptide KTP on the pathological changes induced by an experimental model of sAD in rats.








Similar content being viewed by others
References
Agrawal R, Tyagi E, Shukla R, Nath C (2011) Insulin receptor signaling in rat hippocampus: a study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol 21:261–273
Amani M, Zolghadrnasab M, Salari A-A (2019) NMDA receptor in the hippocampus alters neurobehavioral phenotypes through inflammatory cytokines in rats with sporadic Alzheimer-like disease. Physiol Behav 202:52–61
Angelova H, Pechlivanova D, Dzhambazova E, Landzhov B (2018) Effects of Kyotorphin on the early behavioural and histological changes induced by an experimental model of Alzheimer’s disease in rats. Compt Rend Bulg Acad Sci 71(3):424–430
Arima T, Kitamura Y, Nishiya T, Takagi H, Nomura Y (1996) Kyotorphin (L-tyrosyl-l-arginine) as a possible substrate for inducible nitric oxide synthase in rat glial cells. Neurosci Lett 212:1–4
Arima T, Kitamura Y, Nishiya T, Taniguchi T, Takagi H, Nomura Y (1997) Effects of kyotorphin (l-tyrosyl-l-arginine) ON[3H]NG-nitro-l-arginine binding to neuronal nitric oxide synthase in rat brain. Neurochem Int 30(6):605–611
Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM (2002) Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv 2:363–375
Banks WA, Owen JB, Erickson MA (2012) Insulin in the brain: there and back again. Pharmacol Ther 136(1):82–93
Barker GRI, Warburton EC (2011) When is the hippocampus involved in recognition memory? J Neurosci 31(29):10721–10731
Blokland A, Jolles J (1993) Spatial learning deficit and reduced hippocampal ChAT activity in rats after an ICV injection of streptozotocin. Pharmacol Biochem Behav 44:491–494
Bocheva AI, Dzambazova-Maximova EB (2004) Effects of kyotorphin and analogues on nociception and pentylenetetrazole seizures. Folia Med (Plovdiv) 46(1):40–44
Chen Y, Liang Z, Blanchard J, Dai C-L, Sun S, Lee MH, Grundke-Iqbal I, Iqbal K, Liu F, Gong CX (2013) A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol 47(2):711–725
Conceição K, Magalhães PR, Campos SR, Domingues MM, Ramu VG, Michalek M, Bertani P, Baptista AM, Heras M, Bardaji ER, Bechinger B, Ferreira ML, Castanho MA (2016) The anti-inflammatory action of the analgesic kyotorphin neuropeptide derivatives: insights of a lipid-mediated mechanism. Amino Acids 48(1):307–318
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406
de la Monte SM, Re E, Longato L, Tong M (2012) Dysfunctional pro-ceramide, ER stress, and insulin/IGF signaling networks with progression of Alzheimer’s Disease. J Alzheimers Dis 30(02):S217–S229
de la Torre JC, Stefano GB (2000) Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res Brain Res Rev 34(3):119–136
de Oliveira JS, Abdalla FH, Dornelles GL, Adefegha SA, Palma TV, Signor C, da Silva Bernardi J, Baldissarelli J, Lenz LS, Magni LP, Rubin MA, Pillat MM, de Andrade CM (2016) Berberine protects against memory impairment and anxiogenic-like behavior in rats submitted to sporadic Alzheimer’s-like dementia: involvement of acetylcholinesterase and cell death. Neuro Toxicol 57:241–250
Deacon RM, Rawlins JN (2006) T-maze alternation in the rodent. Nat Protoc 1(1):7–12
Dehghan-Shasaltaneh M, Naghdi N, Choopani S, Alizadeh L, Bolouri B, Masoudi-Nejad A, Riazi GH (2016) Determination of the Best Concentration of Streptozotocin to Create a Diabetic Brain Using Histological Techniques. J Mol Neurosci 59(1):24–35
Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX (2009) Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer’s disease. Am J Pathol 175(5):2089–2098
Detour J, Schroeder H, Desor D, Nehlig A (2005) A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia 46(4):499–508
Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA (2008a) Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiol 45(1):36–49
Dillon GM, Qu X, Marcus JN, Dodart J-C (2008b) Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice. Neurobiol Learn Mem 90(2):426–433
Dubey H, Gulati K, Ray A (2018) Amelioration by nitric oxide (NO) mimetics on neurobehavioral and biochemical changes in experimental model of Alzheimer’s disease in rats. Neurotoxicology 66:58–65
Dzambazova E, Landzhov B, Bocheva A, Bozhilova-Pastirova A (2011) Effects of kyotorphin on NADPH-d reactive neurons in rat’s cerebral cortex after acute immobilization stress. Compt Rend Bulg Acad Sci 64(12):1779–1784
El Khoury NB, Gratuze M, Papon M-A, Bretteville A, Planel E (2014) Insulin dysfunction and Tau pathology. Front Cell Neurosci 8:22
España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA (2010) Intraneuronal β-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer’s disease transgenic mice. Biol Psychiatry 67:513–521
Frölich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Türk A, Hoyer S, Zöchling R, Boissl KW, Jellinger K, Riederer P (1998) Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 105:423–438
Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton PH, Rooke KP, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349:704–706
Godlevsky LS, Shandra AA, Mikhaleva II, Vastyanov RS, Mazarati AM (1995) Seizure-protecting effects of kyotorphin and related peptides in an animal model of epilepsy. Brain Res Bull 37(3):223–226
Grieb P (2016) Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol 5:1741–1752
Grünblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S (2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101:757–770
Hellweg R, Nitsch R, Hock C, Jaksch M, Hoyer S (1992) Nerve growth factor and choline acetyltransferase activity levels in the rat brain following experimental impairment of cerebral glucose and energy metabolism. J Neurosci Res 31:479–486
Honey RC, Marshall VJ, Mcgregor A, Futter J, Good M (2007) Revisiting places passed: sensitization of exploratory activity in rats with hippocampal lesions. Q J Exp Psychol (Hove) 60(5):625–634
Hoyer S, Nitsch R, Oesterreich K (1991) Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect 3:1–14
Ivanova NM, Atanasova D, Pechlivanova DM, Mitreva R, Lazarov N, Stoynev AG, Tchekalarova JD (2015) Long-term intracerebroventricular infusion of angiotensin II after kainate-induced status epilepticus: effects on epileptogenesis, brain damage, and diurnal behavioral changes. Epilepsy Behav 51:1–12
Kinnavane L, Albasser MM, Aggleton JP (2015) Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res 15(285):67–78
Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, Moser MB (2002) Reduced fear expression after lesions of the ventral hippocampus. PNAS 99(16):10825–10830
Lannert H, Hoyer S (1998) Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci 112:1199–1208
Lee Y, Kim YH, Park SJ, Huh JW, Kim SH, Kim SU, Kim JS, Jeong KJ, Lee KM, Hong Y, Lee SR, Chang KT (2014) Insulin/IGF signaling-related gene expression in the brain of a sporadic Alzheimer’s diseasemonkeymodel induced by intracerebroventricular injection of streptozotocin. J Alzheimers Dis 38:251–267
Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte S (2006) Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis 9:13–33
Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE (1995) Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269:973–977
Liu P, Zou LB, Wang LH, Jiao Q, Chi TY, Ji XF, Jin G (2014) Xanthoceraside attenuates tau hyperphosphorylation and cognitive deficits in intracerebroventricular-streptozotocin injected rats. Psychopharmacology 231:345–356
Lowry OH, Rosenbrough HJ, Faar AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mayer G, Nitsch R, Hoyer S (1990) Effects of changes in peripheral and cerebral glucose metabolism on locomotor activity, learning and memory in adult male rats. Brain Res 532:95–100
Mehla J, Pahuja M, Gupta YK (2013) Streptozotocin-induced sporadic Alzheimer’s disease: selection of appropriate dose. J Alzheimers Dis 33:17–21
Nazarenko IV, Zvrushchenko MSh, Volkov AV, Kamenskiĭ AA, Zaganshin RKh (1999) Functional-morphologic evaluation of the effect of the regulatory peptide kyotorphin on the status of the CNS in the post-resuscitation period. Patol Fiziol Eksp Ter 2:31–33
Nazem A, Sankowski R, Bacher M, Al-Abed Y (2015) Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflammation 12:74
Norris PJ, Faull RL, Emson PC (1996) Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer’s disease brains. Brain Res Mol Brain Res 41(1–2):36–49
Paxinos G, Watson C (1998) The rat brain, 4th edn. Academic Press, Elsevier Science
Pedersen NL, Gatz M, Berg S, Johansson B (2004) How heritable is Alzheimer’s disease late in life? Findings from Swedish twins. Ann Neurol 55:180–185
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24(3):525–529
Peng D, Pan X, Cui J, Ren Y, Zhang J (2013) Hyperphosphorylation of tau protein in hippocampus of central insulin-resistant rats is associated with cognitive impairment. Cell Physiol Biochem 32:1417–1425
Perazzo J, Lima C, Heras M, Bardají E, Lopes-Ferreira M, Castanho M (2017) Neuropeptide kyotorphin impacts on lipopolysaccharide-induced glucocorticoid-mediated inflammatory response. A molecular link to nociception, neuroprotection, and anti-inflammatory action. ACS Chem Neurosci 8:1663–1667
Pezze MA, Marshall HJ, Fone KC, Cassaday HJ (2015) Dopamine D1 receptor stimulation modulates the formation and retrieval of novel object recognition memory: role of the prelimbic cortex. Eur Neuropsychopharmacol 25(11):2145–2156
Plaschke K, Hoyer S (1993) Action of the diabetogenic drug streptozotocin on glycolytic and glycogenolytic metabolism in adult rat brain cortex and hippocampus. Int J Dev Neurosci 11:477–483
Porter VR, Buxton WG, Fairbanks LA, Strickland T, O’Connor SM, Rosenberg-Thompson S, Cummings JL (2003) Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. J Neuropsychiatry Clin Neurosci 15(2):180–186
Rodgers SP, Born HA, Das P, Jankowsky JL (2012) Transgenic APP expression during postnatal development causes persistent locomotor hyperactivity in the adult. Mol Neurodegener 7:28
Sá Santos S, Santos SM, Pinto AR, Ramu VG, Heras M, Bardaji E, Tavares I, Castanho MA (2016) Amidated and ibuprofen-conjugated kyotorphins promote neuronal rescue and memory recovery in cerebral hypoperfusion dementia model. Front Aging Neurosci 26(8):1
Sachdeva AK, Misra S, Pal Kaur I, Chopra K (2015) Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: behavioral and biochemical evidence. Eur J Pharmacol 747:132–140
Salkovic-Petrisic M, Osmanovic-Barilar J, Brückner MK, Hoyer S, Arendt T, Riederer P (2011) Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm 118:765–772
Sansbury BE, Hill BG (2014) Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 73:383–399
Santalova IM, Mavlyutov TA, Moshkov DA (2004) Morphofunctional changes in Mauthner neurons during exposure to the neuropeptide kyotorphin. Neurosci Behav Physiol 34(4):327–332
Santos SM, Garcia-Nimo L, Sá Santos S, Tavares I, Cocho JA, Castanho MA (2013) Neuropeptide kyotorphin (tyrosy l-arginine) has decreased levels in the cerebro-spinal fluid of Alzheimer’s disease patients: potential diagnostic and pharmacological implications. Front Aging Neurosci 5:68
Saxena G, Patro IK, Nath C (2011) ICV STZ induced impairment in memory and neuronal mitochondrial function: a protective role of nicotinic receptor. Behav Brain Res 224(1):50–57
Senzai Y (2019) Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neurosci Res 140:43–52
Sharma M, Gupta YK (2001) Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci 68:1021–1029
Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375:754–760
Shingo AS, Kanabayashi T, Kito S, Murase T (2013) Intracerebroventricular administration of an insulin analogue recovers STZ-induced cognitive decline in rats. Behav Brain Res 241:105–111
Shiomi H, Kuraishi Y, Ueda H, Harada Y, Amano H, Takagi H (1981) Mechanism of kyotorphin-induced release of Met-enkephalin from guinea pig striatum and spinal cord. Brain Res 221(1):161–169
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16(4):435–452
Summy-Long JY, Bui V, Gestl S, Koehler-Stec E, Liu H, Terrell ML et al (1998) Effects of central injection of kyotorphin and L-arginine on oxytocin and vasopressin release and blood pressure in conscious rats. Brain Res Bull 45:395–403. https://doi.org/10.1016/S0361-9230(97)00341-9
Tsukahara T, Yamagishi S, Neyama H, Ueda H (2018) Tyrosyl-tRNA synthetase: a potential kyotorphin synthetase in mammals. Peptides 101:60–68
Ueda H, Shiomi H, Takagi H (1980) Regional distribution of a novel analgesic dipeptide kyotorphin (Tyr-Arg) in the rat brain and spinal cord. Brain Res 198(2):460–464
Ueda H, Yoshihara Y, Misawa H, Fukushima N, Katada T, Ui M, Takagi H, Satoh M (1989) The kyotorphin (tyrosine-arginine) receptor and a selective reconstitution with purified Gi, measured with GTPase and phospholipase C assays. J Biol Chem 264(7):3732–3741
Vloeberghs E, Van Dam D, Franck F, Staufenbiel M, de Deyn PP (2007) Mood and male sexual behaviour in the APP23 model of Alzheimer’s disease. Behav Brain Res 180:146–151
Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL (2017) Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol 4:311–322
Wolfe CM, Fitz NF, Nam KN, Lefterov I, Koldamova R (2019) The Role of APOE and TREM2 in Alzheimer’s disease current understanding and perspectives. Int J Mol Sci 20:81
Yang Y, Mailman RB (2018) Strategic neuronal encoding in medial prefrontal cortex of spatial working memory in the T-maze. Behav Brain Res 343:50–60
Acknowledgements
The research was supported by Grant 18/2016 of the Medical University of Sofia, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved by Bulgarian Food Safety Agency (No 144/04.08.2021) that is in accordance with EC Directive 2010/63/EU for animal experiments.
Additional information
Handling Editor: V. Bolshakov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angelova, H., Pechlivanova, D., Krumova, E. et al. Moderate protective effect of Kyotorphin against the late consequences of intracerebroventricular streptozotocin model of Alzheimer’s disease. Amino Acids 51, 1501–1513 (2019). https://doi.org/10.1007/s00726-019-02784-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02784-5