Abstract
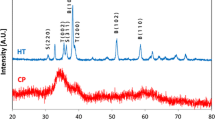
Polycrystalline material of a sulfate apatite with chemical composition Na6Ca4(SO4)6F2 or (Na2Ca4)Na4(SO4)6F2 has been synthesized by solid state reactions. Basic crystallographic data are as follows: hexagonal symmetry, a = 9.3976(1) Å, c = 6.8956(1) Å, V = 527.39(1) Å3, Z = 1, space group P63/m. For structural investigations the Rietveld method was employed. Thermal expansion has been studied between 25 and 600 °C. High temperature (HT) powder diffraction data as well as thermal analysis indicate that the apatite-type compound undergoes a reconstructive phase transition in the range between 610 and 630 °C. Single-crystals of the HT-polymorph were directly grown from the melt. Structural investigations based on single-crystal diffraction data of the quenched crystals performed at −100 °C showed orthorhombic symmetry (space group Pna21) with a = 12.7560(8) Å, b = 8.6930(4) Å, c = 9.8980(5) Å, V = 1097.57(10) Å3 and Z = 2. Unit cell parameters for a quenched polycrystalline sample of the HT-form obtained at ambient conditions from a LeBail-fit are as follows: a = 12.7875(1) Å, b = 8.7255(1) Å, c = 9.9261(1) Å, V = 1107.53(2) Å3. The lattice parameters of both modifications are related by the following approximate relationships: a HT ≈ 2c RT, b HT ≈ -(½a RT + b RT), c HT ≈ a RT. The HT-modification is isotypic with the corresponding potassium compound K6Ca4(SO4)6F2. The pronounced disorder of the sulphate group even at low temperatures has been studied by maximum entropy calculations. Despite the first-order character of the transformation clusters of sulfate groups surrounding the fluorine anions can be identified in both polymorphs. Each of the three next neighbor SO4-tetrahedra within a cluster is in turn surrounded by 8–9 M-cations (M: Na,Ca) defining cage-like units. However, in the apatite structure the corresponding three tricapped trigonal prisms are symmetry equivalent. Furthermore, the central fluorine atom of each cluster is coordinated by three next M-neighbors (FM3-triangles), whereas in the HT-polymorph a four-fold coordination is observed (FM4-tetrahedra).










Similar content being viewed by others
References
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla MC, Polidori G, Camalli M (1994) SIR92 - a program for automatic solution of crystal structures by direct methods. J Appl Crystallogr 27:435
Apella MC, Baran EJ (1979) Röntgenographische und IR-spektroskopische Untersuchungen der Substitution von Phosphat- und Sulfat-Ionen im Fluorapatitgitter. Z Naturforsch 34b:1124–1127
Apella MC, Baran EJ (1981) Zur Kristallchemie der gemischten Phosphat/Sulfat Fluorapatite. Z Naturforsch 36b:644–645
Bergerhoff G, Berndt M, Brandenburg K, Degen T (1999) Concerning inorganic crystal structure types. Acta Crystallogr B55:147–156
Cavarretta G, Mottana A, Tecce F (1981) Cesanite, Ca2Na3[(OH)(SO4)3], a sulphate isotypic to apatite, from the Cesano geothermal field (Latium, Italy). Min Mag 44:269–273
De Vries RY, Briels WJ, Feil D (1994) Novel treatment of the experimental data in the application of the maximum-entropy method to the determination of the electron-density distribution from X-ray experiments. Acta Crystallogr A50:383–391
Deganello S, Artioli G (1982) Thermal expansion of cesanite between 22–390 °C. N Jb Mineral Mh 1982:565–568
Demartin F, Gramaccioli CM, Campostrini I, Pilati T (2010) Aiolosite, Na2(Na2Bi)(SO4)3Cl, a new sulfate isotypic to apatite from La Fossa Crater, Vulcano, Aeolian Islands, Italy. Am Mineral 95:382–385
Dowty E (2011) ATOMS, Version 6.4, Shape Software. Kingsport, USA
Fayos J, Watkin DJ, Pérez-Méndez M (1987) Crystal structure of the apatite-like compound K3Ca2(SO4)3F. Am Mineral 72:209–212
Finger LW, Cox DE, Jephcoat AP (1994) A correction for powder diffraction peak asymmetry due to axial divergence. J Appl Crystallogr 27:892–900
Fletcher L (1889) On the crystals of percylite, caracolite and an oxychloride of lead (daviesite), from Mina Beatriz, Sierra Gorda, Atacama, South America. Min Mag 8:171–180
Klement R (1939) Natrium-Calcium-Sulfatapatit Na6Ca4(SO4)6F2. Naturwissenschaften 27:568
McConnell D (1937) The substitution of SiO4- and SO4-groups for PO4-groups in the apatite structure; ellastadite, the end-member. Am Mineral 22:977–986
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Pasero M, Kampf AR, Ferraris C, Pekov IV, Rakovan J, White TJ (2010) Nomenclature of the apatite supergroup minerals. Eur J Mineral 22:163–179
Perret R, Bouillet AM (1975) Les apatites-sulfates Na3Cd2(SO4)3Cl and Na3Pd2(SO4)3Cl. Bull Soc Fr Mineral Cristallogr 8:254–256
Petřiček V, Dušek M, Palatinus L (2006) Jana2006. The crystallographic computing system. Institute of Physics, Praha
Piotrowski A, Kahlenberg V, Fischer RX, Lee Y, Parise JB (2002a) The crystal structures of cesanite and its synthetic analogue – a comparison. Am Mineral 87:715–720
Piotrowski A, Kahlenberg V, Fischer RX (2002b) The solid solution series of the sulfate apatite system Na6.45Ca3.55(SO4)6(FxCl1-x)1.55. J Solid State Chem 163:398–405
Piotrowski A, Kahlenberg V, Fischer RX (2004) Mixed phosphate-sulfate fluor apatites, possible materials in dental fillers. Eur J Mineral 16:279–284
Prince E (ed) (2004) International Tables for Crystallography, Vol. C: Mathematical, physical and chemical tables. Kluwer Academic Publishers, Dordrecht
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic elongation: a quantitative measure of distortion in coordination polyhedra. Science 172:567–570
Rodriguez-Carvajal J (2005) FullProf.2k, version 3.3. Laboratoire Leon Brillouin (CEA-CNRS), France
Roisnel T (2013) WinPlotr, http://www.cdifx.univ-rennes1.fr/winplotr/winplotr.htm. Accessed 08 August 2013.
Schneider W (1967) Caracolit, das Na3Pb2(SO4)3Cl mit Apatitstruktur. N Jb Mineral Mh:58–64
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Shiga Y, Urashima Y (1987) A sodian sulfatian fluorapatite from an epithermal calcite-quartz vein of the Kushikino mine, Kagoshima Prefecture, Japan. Can Mineral 25:673–681
Tasci ES, De la Flor G, Orobengoa D, Capillas C, Perez-Mato JM, Aroyo MI (2012) An introduction to the tools hosted in the Bilbao Crystallographic Server. EJP Web Conf 22:00009
Thakre PS, Gedam SC, Dhoble SJ, Atram RG (2011) Luminescence investigations on sulfate apatite Na6(SO4)2FCl: RE (RE = Dy, Ce or Eu) phosphors. J Lumin 131:2683–2689
Tõnsuaadu K, Peld M, Quarton M, Bender V, Veiderma M (2002) Studies on SO4 2- ion incorporation into apatite structure. Phosphorus Sulfur 177:1873–1876
Triviño-Vázquez F (1985) A new compound: K3Ca2(SO4)3F, identified in coatings of heat recovery cyclones. Cem Concr Res 15:581–584
Van Smaalen S, Palatinus L, Schneider M (2003) The maximum entropy method in superspace. Acta Crystallogr A59:459–569
Websky M (1886) Über Caracolit und Percylit. Sitzber K Preuss Aka 48:1045–1050
Werner PE, Eriksson L, Westdahl M (1985) TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J Appl Crystallogr 18:367–370
White TJ, Dong ZL (2003) Structural derivation and crystal chemistry of apatites. Acta Crystallogr B59:1–16
Acknowledgments
The authors would like to acknowledge the helpful comments and suggestions of Lutz Nasdala (associate editor), Christian L. Lengauer as well as two other anonymous reviewers which improved the quality of this paper. Furthermore, we would like to thank Mrs. Daniela Schmidmair (University of Innsbruck) for troubleshooting graphics software errors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: L. Nasdala
Rights and permissions
About this article
Cite this article
Botta, C., Kahlenberg, V., Hejny, C. et al. Structural investigations, high temperature behavior and phase transition of Na6Ca4(SO4)6F2 . Miner Petrol 108, 487–501 (2014). https://doi.org/10.1007/s00710-013-0319-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-013-0319-x