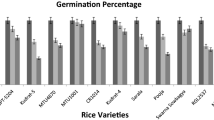
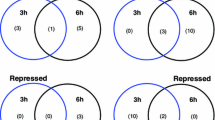
Abstract
Salt stress response includes alteration in the activity of various important enzymes in plants. Nitrate reductase (NR) is one of the known enzyme affected by salt stress. In this study, contrasting salt responsive cultivars (CVS) (IR64-sensitive and CSR 36-tolerant) were considered to study the regulation of NR genes under salt stress conditions. Using Arabidopsis genes Nia1 and Nia2, three different NR genes were identified in rice and their expression study was conducted. Under stress condition, salt-sensitive CVS (IR64) showed a decrease in NR activity under in vitro and in vivo conditions, whereas tolerant CVS showed an increase in NR activity. Different trends for NR activity in contrasting genotype are explained by the variable number of GATA element in the upstream region of the NR gene. This variation of NR activity in contrasting CVS further co-relates with the transcript level of NR genes. The transcript level of three different NR genes also evidenced the effect of CREs in gene regulation. Promoter (1-kb upstream region) of different NR genes contained different abiotic stress-responsive CREs, which explain the differential behavior of these genes towards the abiotic stress. Overall, this study concludes the role of CREs in the regulation of NR gene and indicates the importance of transcriptional control of NR activity under stress condition. This is the first type of report that highlights the role of the regulatory mechanism of NR genes under salt stress condition.






Similar content being viewed by others
Abbreviations
- NR:
-
Nitrate reductase
- CVS:
-
Cultivars
- CREs:
-
Cis-regulatory elements
References
Apse MP, Blumwald E (2002) Engineering salt tolerance in plants. Curr Opin Biotechnol 13(2):146–150
Arshi A, Abdin M, Iqbal M (2002) Growth and metabolism of senna as affected by salt stress. Biol Plant 45(2):295–298
Baki G, Siefritz F, Man HM, Weiner H, Kaldenhoff R, Kaiser W (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23(5):515–521
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59(1):21–39. https://doi.org/10.1146/annurev.arplant.59.032607.092830
Bi Y-M, Zhang Y, Signorelli T, Zhao R, Zhu T, Rothstein S (2005) Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J 44(4):680–692. https://doi.org/10.1111/j.1365-313X.2005.02568.x
Carillo P, Mastrolonardo G, Nacca F, Fuggi A (2005) Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct Plant Biol 32(3):209–219
Cazetta JO, Villela LCV (2004) Nitrate reductase activity in leaves and stems of tanner grass (Brachiaria radicans napper). Sci Agric 61(6):640–648
Chandrasekaran P, Sivakumar R, Nandhitha G, Vishnuveni M, Boominathan P, Senthilkumar M (2017) Impact of PPFM and PGRs on seed germination, stress tolerant index and catalase activity in tomato (Solanum lycopersicum L) under drought. Int J Curr Microbiol App Sci 6(6):540–549
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560
Correia MJ, Fonseca F, Azedo-Silva J, Dias C, David MM, Barrote I, Osório ML, Osório J (2005) Effects of water deficit on the activity of nitrate reductase and content of sugars, nitrate and free amino acids in the leaves and roots of sunflower and white lupin plants growing under two nutrient supply regimes. Physiol Plant 124(1):61–70
Cramer MD, Lips SH (1995) Enriched rhizosphere CO2 concentrations can ameliorate the influence of salinity on hydroponically grown tomato plants. Physiol Plant 94(3):425–432
Daniel-Vedele F, Filleur S, Caboche M (1998) Nitrate transport: a key step in nitrate assimilation. Curr Opin Plant Biol 1(3):235–239
Evans HJ, Nason A (1953) Pyridine nucleotide-nitrate reductase from extracts of higher plants. Plant Physiol 28(2):233
Ferrario-Méry S, Valadier M-H, Foyer CH (1998) Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol 117(1):293–302
Flores P, Botella M, Martinez V, Cerdá A (2000) Ionic and osmotic effects on nitrate reductase activity in tomato seedlings. J Plant Physiol 156(4):552–557
Flores P, Angeles Botella M, Martínez V, Cerdá A (2002) Response to salinity of tomato seedlings with a split-root system: nitrate uptake and reduction. J Plant Nutr 25(1):177–187
Foyer C, Zhang H (2011) Annual plant reviews, nitrogen metabolism in plants in the post-genomic era, vol 42. John Wiley & Sons
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gouia H, Ghorbal MH, Touraine B (1994) Effects of NaCl on flows of N and mineral ions and on NO3-reduction rate within whole plants of salt-sensitive bean and salt-tolerant cotton. Plant Physiol 105(4):1409–1418
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51(1):463–499
Jamil A, Riaz S, Ashraf M, Foolad M (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30(5):435–458
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43(6):1274–1279
Katiyar S, Dubey R (1992) Influence of NaCl salinity on behaviours of nitrate reductase and nitrite reductase in rice seedlings differing in salt tolerance. J Agron Crop Sci 169(5):289–297
Kleinhofs A, Chao S, Sharp P (1988) Mapping of nitrate reductase genes in barley and wheat
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
López-Ochoa L, Acevedo-Hernández G, Martínez-Hernández A, Argüello-Astorga G, Herrera-Estrella L (2007) Structural relationships between diverse cis-acting elements are critical for the functional properties of a rbcS minimal light regulatory unit. J Exp Bot 58(15–16):4397–4406
Loussaert DF, O'neill D, Simmons CR, Wang H (2010) Nitrate reductases from red algae, compositions and methods of use thereof. Google Patents,
Lutts S, Kinet J, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78(3):389–398
Meloni DA, Gulotta MR, Martínez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16(1):39–46
Misra N, Dwivedi U (1990) Nitrogen assimilation in germinating Phaseolus aureus seeds under saline stress. J Plant Physiol 135(6):719–724
Nath M, Yadav S, Kumar Sahoo R, Passricha N, Tuteja R, Tuteja N (2016) PDH45 transgenic rice maintain cell viability through lower accumulation of Na(+), ROS and calcium homeostasis in roots under salinity stress. J Plant Physiol 191:1–11. https://doi.org/10.1016/j.jplph.2015.11.008
Ouyang B, Yang T, Li H, Zhang L, Zhang Y, Zhang J, Ye Z (2007) Identification of early salt stress response genes in tomato root by suppression subtractive hybridization and microarray analysis. J Exp Bot 58(3):507–520
Passricha N, Saifi S, Ansari MW, Tuteja N (2016a) Prediction and validation of cis-regulatory elements in 5′ upstream regulatory regions of lectin receptor-like kinase gene family in rice. Protoplasma. https://doi.org/10.1007/s00709-016-0979-6
Passricha N, Saifi S, Khatodia S, Tuteja N (2016b) Assessing zygosity in progeny of transgenic plants: current methods and perspectives. J Biol Methods 3(3):e46
Passricha N, Saifi S, Khatodia S, Tuteja N (2016c) Assessing zygosity in progeny of transgenic plants: current methods and perspectives. J Biol Methods; Vol 3, No 3 (2016)
Passricha N, Saifi SK, Gill SS, Tuteja R, Tuteja N (2019a) Chapter 3 - role of plant helicases in imparting salinity stress tolerance to plants. In: Tuteja R (ed) Helicases from All Domains of Life. Academic Press, pp 39–52. https://doi.org/10.1016/B978-0-12-814685-9.00003-8
Passricha N, Saifi SK, Kharb P, Tuteja N (2019b) Marker-free transgenic rice plant overexpressing pea LecRLK imparts salinity tolerance by inhibiting sodium accumulation. Plant Mol Biol. https://doi.org/10.1007/s11103-018-0816-8
Peuke AD, Glaab J, Kaiser WM, Jeschke WD (1996) The uptake and flow of C, N and ions between roots and shoots in Ricinus communis L. IV. Flow and metabolism of inorganic nitrogen and malate depending on nitrogen nutrition and salt treatment. J Exp Bot 47(3):377–385
PLAUT Z (1974) Nitrate reductase activity of wheat seedlings during exposure to and recovery from water stress and salinity. Physiol Plant 30(3):212–217
Raghuram N, Chandok MR, Sopory SK (1999) Light regulation of nitrate reductase gene expression in maize involves a G-protein. Mol Biol Res Commun 2(2):86–90
Rao RK, Gnanam A (1990) Inhibition of nitrate and nitrite reductase activities by salinity stress in Sorghum vulgare. Phytochemistry 29(4):1047–1049
Redinbaugh MG, Campbell WH (1981) Purification and characterization of NAD (P) H: nitrate reductase and NADH: nitrate reductase from corn roots. Plant Physiol 68(1):115–120
Sagi M, Savidov N, L'vov N, Lips S (1997) Nitrate reductase and molybdenum cofactor in annual ryegrass as affected by salinity and nitrogen source. Physiol Plant 99(4):546–553
Saifi SK, Passricha N, Swain DM, Tuteja N (2017) Prediction of cis-regulatory elements for a detailed insight of RuvB family genes from Oryza sativa. Oryza 54(2):135–147
Saifi SK, Passricha N, Tuteja R, Tuteja N (2018) Stress-induced Oryza sativa RuvBL1a is DNA-independent ATPase and unwinds DNA duplex in 3′ to 5′ direction. Protoplasma 255(2):669–684. https://doi.org/10.1007/s00709-017-1178-9
Sazegari S, Niazi A, Ahmadi FS (2015) A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes. Bioinformation 11(2):101
Sepehr MF, Ghorbanli M, Amini F (2012) The effect of water stress on nitrate reductase activity and nitrogen and phosphorus contents in Cuminum cyminum L. Pak J Bot 44(3):899–903
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112(7):1209–1221
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32(4):237–249
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Srivastava H (1980) Regulation of nitrate reductase activity in higher plants. Phytochemistry 19(5):725–733
Teakle GR, Manfield IW, Graham JF, Gilmartin PM (2002) Arabidopsis thaliana GATA factors: organisation, expression and DNA-binding characteristics. Plant Mol Biol 50(1):43–56
Viégas RA, de Melo AB, da Silveira JG (1999) Nitrate reductase activity and proline accumulation in cashew in response to NaCl salt shock. Revista Brasileira de Fisiologia Vegetal (Brazil)
Wang W-X, Pelah D, Alergand T, Shoseyov O, Altman A (2002) Characterization of SP1, a stress-responsive, boiling-soluble, homo-oligomeric protein from aspen. Plant Physiol 130(2):865–875
Wilson I, Bright J, Hancock J, Harrison J, Neill S, Ribeiro D, Barros R, Desikan R, Morris P (2008) Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59(2):165–176. https://doi.org/10.1093/jxb/erm293
Yadav S, Gill SS, Passricha N, Gill R, Badhwar P, Anjum NA, Francisco J-BJ, Tuteja N (2018) Genome-wide analysis and transcriptional expression pattern-assessment of superoxide dismutase (SOD) in rice and Arabidopsis under abiotic stresses. Plant Gene. https://doi.org/10.1016/j.plgene.2018.10.001
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10(12):615–620
You J, Zhang L, Song B, Qi X, Chan Z (2015) Systematic analysis and identification of stress-responsive genes of the NAC gene family in Brachypodium distachyon. PLoS One 10(3):e0122027
Zhao C-R, Ikka T, Sawaki Y, Kobayashi Y, Suzuki Y, Hibino T, Sato S, Sakurai N, Shibata D, Koyama H (2009) Comparative transcriptomic characterization of aluminum, sodium chloride, cadmium and copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biol 9(1):32. https://doi.org/10.1186/1471-2229-9-32
Acknowledgments
This research work is financially supported by a fellowship grant from the University Grant Commission-Basic Scientific Research (UGC BSR) (F.25-1/2013-14 (BSR)/7-371/2012) to Ms. Pooja Rohilla and the University Grant Commission- Special Assistance Programme (UGC-SAP) (F.20/2012(SAP-II)) grant from UGC, New Delhi. Authors are also thankful to Dr. Nishat Passricha, ICGEB, New Delhi, for the help in the conducting of qPCR experiments.
Author information
Authors and Affiliations
Contributions
RP has contributed to the idea and designed the experiments. YJP is a mentor and edited the manuscript. Both authors thoroughly read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key message: Under salinity stress Nitrate reductase regulated by transcriptional machinery with the help of CREs
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Rohilla, P., Yadav, J.P. Acute salt stress differentially modulates nitrate reductase expression in contrasting salt responsive rice cultivars. Protoplasma 256, 1267–1278 (2019). https://doi.org/10.1007/s00709-019-01378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01378-y