Abstract
The biological nature, ultrastructure, distribution, and mode of transmission between generations of the microorganisms associated with three species (Orthezia urticae, Matsucoccus pini, Steingelia gorodetskia) of primitive families (archaeococcoids = Orthezioidea) of scale insects were investigated by means of microscopic and molecular methods. In all the specimens of Orthezia urticae and Matsucoccus pini examined, bacteria Wolbachia were identified. In some examined specimens of O. urticae, apart from Wolbachia, bacteria Sodalis were detected. In Steingelia gorodetskia, the bacteria of the genus Sphingomonas were found. In contrast to most plant sap-sucking hemipterans, the bacterial associates of O. urticae, M. pini, and S. gorodetskia are not harbored in specialized bacteriocytes, but are dispersed in the cells of different organs. Ultrastructural observations have shown that bacteria Wolbachia in O. urticae and M. pini, Sodalis in O. urticae, and Sphingomonas in S. gorodetskia are transovarially transmitted from mother to progeny.
Similar content being viewed by others
Introduction
Scale insects (coccoids) constitute the infraorder Coccomorpha within the hemipteran suborder Sternorrhyncha (Williams and Hodgson 2014). There are estimated to be about 8000 species of scale insects. Many coccoid species are considered to be economically important pests in horticulture, agriculture, and forestry (Gullan and Cook 2007; Gullan and Martin 2009). Scale insects are more diverse in terms of their morphology, chromosome systems, modes of reproduction (parthenogenesis, hermaphroditism, bisexual reproduction), and types of symbioses than any of the other sternorrhynchan groups (Gullan and Martin 2009).
Scale insects are usually divided into two groups: Orthezioidea Amyot et Serville, 1843 and Coccoidea Fallen, 1814 which are considered as superfamilies (Koteja 1974; Danzig 1980; Williams and Watson 1990; Morales 1991; Kosztarab 1996; Ben-Dov 2005; Gavrilov-Zimin 2018). Other researchers treat these groups as informal archaeococcoids and neococcoids (Cook et al. 2002; Foldi 2005; Hodgson and Foldi 2006; Hardy et al. 2008; Kaydan and Kozár 2010; Williams et al. 2011; Hodgson 2012, 2014; Hodgson and Hardy 2013). In the present work, we have investigated symbiotic systems of three species of archaeococcoids (= Orthezioidea): Orthezia urticae (Linnaeus, 1758), Matsucoccus pini (Green, 1925), and Steingelia gorodetskia Nasonov, 1908.
Orthezia urticae is a species which belongs to the family Ortheziidae, which has been considered to be one of the oldest families of scale insects (Koteja 1996; Kozár and Miller 2000; Vea and Grimaldi 2012).
The genera Matsucoccus Cockerell 1909 and Steingelia Nasonov 1908 have been ascribed to the family Margarodidae (e.g., Morrison 1928; Kosztarab and Kozár 1988; Ben-Dov 2005; Kozár et al. 2013; Gavrilov-Zimin 2018). Koteja (1974) proposed a phylogeny and classification of the scale insects that gave family rank to a number of groups that were previously placed within Margarodidae sensu lato, e.g., Matsucoccidae. The results of studies conducted by several authors have supported Koteja’s classification (e.g., Foldi 2004, 2005; Booth and Gullan 2006; Hodgson and Foldi 2006; Ben-Dov 2012; Hodgson and Hardy 2013; Mech et al. 2013; Wang et al. 2016). The genus Steingelia was initially placed in the family Kuwaniidae (Koteja 1974; Dziedzicka 1977), but was later assigned its own family, Steingeliidae (e.g., Koteja 1996, 2000).
Most scale insects, like many other hemipterans which feed on plant sap that is lacking essential amino acids, are host to obligate symbiotic microorganisms (reviewed in Buchner 1965; Tremblay 1977; Douglas 1989, 1998; Baumann 2005; Rosenblueth et al. 2018). Previous studies based on paraffin technique have revealed that scale insects, as opposed to remaining Sternorrhyncha (aphids, whiteflies, psyllids), are characterized by an enormous diversity of symbiotic associates (reviewed in Walczuch 1932; Buchner 1965; Tremblay 1977). More recent ultrastructural and molecular analyses have confirmed that the symbioses of scale insects are much more diverse than those in the remaining Sternorrhyncha, with respect to the systematic affiliation of symbionts, distribution in the host insect body, and the mode of transmission from the mother to the progeny (Fukatsu and Nikoh 2000; von Dohlen et al. 2001; Thao et al. 2002; Szklarzewicz et al. 2006, 2013, 2018; Niżnik and Szklarzewicz 2007; Kono et al. 2008; Matsuura et al. 2009; Gruwell et al. 2010, 2012; Ramirez-Puebla et al. 2010; Gatehouse et al. 2011; McCutcheon and von Dohlen 2011; Vashishtha et al. 2011; Dhami et al. 2012; Rosenblueth et al. 2012, 2018; Husnik et al. 2013; Koga et al. 2013a; Sabree et al. 2013; Rosas-Pérez et al. 2014; Michalik et al. 2016, 2018; Szabo et al. 2017). Several families of scale insects, e.g., Steingeliidae, Xylococcidae, Matsucoccidae, Kermesidae, Kuwaniidae, Dactylopiidae, were regarded as asymbiotic (Buchner 1965; Tremblay 1977); however, the results of recent ultrastructural or molecular studies have revealed that some of them, i.e., Steingeliidae, Dactylopiidae, Kermesidae, and Matsucoccidae, may harbor bacterial or yeast-like associates (Koteja et al. 2003; Ramirez-Puebla et al. 2010; Szklarzewicz et al. 2014; Podsiadło et al. 2018; Rosenblueth et al. 2018). In contrast to the neococcoids, which were the object of numerous molecular analyses, the symbiotic systems of archaeococcoids are not well known. Taking the above into consideration, the aim of the present study was to re-examine the representatives of the archaeococcoid families Ortheziidae, Steingeliidae, and Matsucoccidae and provide information on their microbiota.
Material and methods
Insects
Orthezia urticae (Linnaeus, 1758) (Fig. S1a) is a polyphagous pest of herbaceous plants which prefers the stinging nettle Urtica dioica. O. urticae develops one generation yearly (Kosztarab and Kozár 1988). The adult females of O. urticae were collected from the stems of U. dioica in May and June of the years 1994, 1995, 2015, and 2016 in Kraków (located in the south of Poland).
Steingelia gorodetskia Nasonov, 1908 (Fig. S1b) is a monophagous species which resides on the roots of birch trees. The life cycle of S. gorodetskia lasts 1 year (Koteja and Żak-Ogaza 1981). The larvae live on roots situated about 20 cm below the ground’s surface. Adult females migrate to the ground’s surface, where they lay eggs. The larvae of the last instar of S. gorodetskia were collected from the roots of the birch Betula verrucosa in April 2004 in Kraków. The adult females were collected from dry, fallen leaves of the birch B. verrucosa in May and June 2001, 2002, 2004, and 2016–2018 in Kraków and May and June 2016 in Kobiór (both localities in the southern region of Poland).
Matsucoccus pini (Green, 1925) (Fig. S1c), like other species of Matsucoccidae family, is a serious pest for pine forests (Foldi 2004). M. pini develops two generations a year (Siewniak 1976). The females of M. pini were collected from the bark crevices of the pine Pinus sylvestris in May 2012 and 2013 in Kuźnia Raciborska (located in the south of Poland).
Molecular analyses
The specimens of S. gorodetskia, M. pini, and O. urticae designated for molecular analyses were fixed in 100% ethanol. Before DNA extraction, the specimens were placed in 5% sodium hypochlorite for 1 min and then rinsed in distillated water three times for 1 min. DNA was isolated from 20 individuals of S. gorodetskia, 20 of O. urticae, and 20 of M. pini using Sherlock AX extraction kit (A&A Biotechnology) abiding by manufacturer protocol. The identification of bacterial associates of the species examined was done on the basis of the sequences of their 16S rRNA genes. The 16S rRNA gene was amplified using universal, eubacterial primers: 8F and 1541R, and following this, the purified products were cloned into pJET1.2/blunt plasmid vector using a Clone JET PCR Cloning Kit (Thermo Scientific). The ligated mixtures were transformed into component cells Escherichia coli TOP10F. After 16 h of incubation in 37 °C, the occurrence of amplified 16S rRNA genes was confirmed through diagnostic PCR reactions with primers: pJET For and pJET Rev. (Thermo Scientific). For each species, 50 positive colonies were subjected to restriction fragment analysis (RFLP) using the MspI restrictive enzyme (Thermo Scientific). After this, selected colonies were incubated in liquid LB media with ampicillin (A&A Biotechnology) and then plasmids were isolated using Plasmid Mini AX Kit (A&A Biotechnology) and sequenced. Molecular cloning was repeated for each of the species examined. Additionally, 20 specimens of M. pini and 20 specimens of O. urticae were screened for the presence of Wolbachia (M. pini), as well as Wolbachia- and Sodalis-like (O. urticae) symbionts, using PCR reactions with Wolbachia- and Sodalis-specific primers (Fukatsu and Nikoh 1998; Zhou et al. 1998) under the following conditions: an initial denaturation step at 94 °C for a duration of 3 min, followed by 33 cycles at 94 °C for 30 s, 55 °C (Wolbachia) or 54 °C (Sodalis) for 40 s, 70 °C for 1 min 40 s, and a final extension step of 5 min at 72 °C. The PCR products were made visible through electrophoresis in 1.5% agarose gel stained with Midori Green (Nippon Genetics Europe), and subsequently sequenced (Genomed). The nucleotide sequences obtained were deposited into the GenBank database under the following accession numbers: MK462262–MK462265.
Phylogenetic analyses
The phylogenetic analysis was performed based on sequences of 16S rRNA gene of S. gorodetskia symbiont and selected representatives of the Alphaproteobacteria phylum. The sequences, homologous to the sequence obtained, were found in the GenBank database using CLC MainWorkbench 7 software. The sequences were then edited using BioEdit Sequence Alignment Editor 5.0.9 (Hall 1999), and following this, the sequence alignments were generated using ClustalX 1.8 (Thompson et al. 1997). The base composition was estimated using MEGA 7.0. software (Kumar et al. 2016). The phylogenetic analysis was conducted by maximum likelihood (ML) and neighbor joining (NJ) methods using MEGA 7.0. software (Kumar et al. 2016).
Light (LM) and electron microscopy (TEM)
The dissected abdomens of females of O. urticae, S. gorodetskia, and M. pini were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at room temperature for a period of 3 months. The material was then rinsed in the same buffer with an addition of sucrose (5.8 g/100 mL), postfixed for 1.5 h in 1% osmium tetroxide, dehydrated in a graded series of ethanol and acetone, and embedded in epoxy resin Epon 812 (Serva, Heidelberg, Germany). Semithin sections were stained with 1% methylene blue in 1% borax and photographed under a Nikon Eclipse 80i light microscope. Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined in a JEM 100 SX EM and Jeol JEM 2100 transmission electron microscopes at 80 kV.
Results
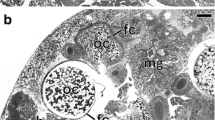
Ultrastructural analyses revealed that in the fat body cells (Fig. 1a), gut epithelium, in the ovarioles (i.e., structural and functional units constituting insect ovaries), and cells of the lateral oviduct of all the examined specimens of Orthezia urticae, numerous small, rod-shaped bacteria with a mean diameter of 0.5 μm and length of 1.9 μm are present. The highest concentration of these bacteria has been observed in fat body and in ovarioles. Within the ovarioles, the bacteria are dispersed throughout all the cells: in follicular cells (Fig. 1c), oocytes (Fig. 1c) and trophocytes (not shown) (for the ovariole organization in scale insects, see Fig. 1d). In ovarioles of some specimens aside from small bacteria, large, elongated bacteria were observed (Fig. 1b). The latter are approximately 1 μm in diameter and are significantly less numerous than the small, rod-shaped bacteria. The comparison of ultrastructural and molecular results indicates that the smaller microorganisms represent bacteria Wolbachia, whereas the larger ones belong to the genus Sodalis. Screen PCR reactions using symbiont specific primers have revealed that bacteria Wolbachia occur in all 20 specimens examined, whereas Sodalis-like symbionts were detected only in 9 out of 20 individuals. The comparison of the obtained 16S rRNA gene sequences of Wolbachia, as well as Sodalis-like symbionts (respectively), has indicated that they are identical. The sequence of 16S rRNA gene of Wolbachia displays a high similarity (99%) to the 16S rRNA gene of bacteria Wolbachia occurring in the body of beetles belonging to the genus Diabrotica [AY007550, AY007548, AY007447]. In turn, the sequence of 16S rRNA gene of Sodalis-like symbiont detected in some specimens of O. urticae shows a high similarity (98%) to Sodalis bacteria associated with the weevil Curculio hachijoensis [AB746396].
Distribution of symbiotic bacteria in Orthezia urticae (Ortheziidae). a Bacteria Wolbachia (Wb) in the cytoplasm of fat body cell. TEM, scale bar = 1 μm. b Bacteria Sodalis (S) in the cytoplasm of follicular cell. Follicular cell nucleus (fn); mitochondrium (m). TEM, scale bar = 2 μm. c Bacteria Wolbachia (Wb) in the cytoplasm of the follicular cell (fc) and the oocyte (oc). Mitochondrium (m). TEM, scale bar = 1 μm. d Ovariole (longitudinal section). Follicular cells (fc); nutritive cord (nc); oocyte (oc); trophocyte (tc); trophic core (asterisk). LM, scale bar = 40 μm
Ultrastructural observations indicated that in the body of Matsucoccus pini, small, rod-shaped bacteria measuring about 0.5 μm in diameter and 1 μm in length are present (Fig. 2a–c). Just as in O. urticae, the bacteria are distributed in different organs: in the fat body cells, in gut epithelium, and in the ovaries (Fig. 2a–c). Since molecular analyses showed that in the body of M. pini, only bacteria Wolbachia are present, the small, rod-shaped microorganisms represent this species. All the obtained sequences of 16S rRNA genes of examined individuals of M. pini are identical and show a 99% similarity to the homologous sequences isolated from Drosophila incompta [CP011148] and Drosophila simulans [CP001391].
Distribution of symbiotic bacteria in Matsucoccus pini (Matsucoccidae). a Bacteria Wolbachia (Wb) in the trophocyte cytoplasm. Mitochondrium (m). TEM, scale bar = 2 μm. b Bacterium Wolbachia (Wb) migrating through the trophic core to the oocyte. Microtubules (arrows); mitochondrium (m). TEM, scale bar = 1 μm. c Bacteria Wolbachia (Wb) in the oocyte cytoplasm. Mitochondrium (m). TEM, scale bar = 1 μm
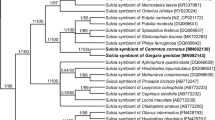
Numerous small, rod-shaped bacteria are present in the fat body cells, the gut epithelium, ovaries, and the cells of the lateral oviduct of all the individuals of Steingelia gorodetskia (Fig. 3a–d) that were collected in two different locations. The highest amount of these bacteria was observed in the ovary: in the cystocytes (i.e., undifferentiated germ cells) constituting larval ovaries (Fig. 3a) as well as in the ovarioles of adult females (Fig. 3b–d): in trophocytes, in the cells of the inner epithelial sheath, in oocytes, in follicular cells surrounding the developing oocytes, in trophic core and nutritive cords. These microorganisms possess several electron-translucent areas within the cytoplasm and have measurements of about 0.4 μm in diameter and 1.4 μm in length. Neither the ultrastructural observations nor the molecular analyses revealed the presence in the body of S. gorodetskia of other species of bacteria. Molecular phylogenetic analyses based on the sequence of 16S rRNA gene indicated that bacteria present in S. gorodetskia belong to the Sphingomonadales order within Alphaproteobacteria phylum and are closely related to the soil bacterium Sphingomonas echinoides (Fig. 4). The length of the sequences used in the phylogenetic analysis was 1297 bp, whereas the composition of the nucleotide was approximately equal: 21.2% T, 23.1% C, 24.8% A, and 30.9% G.
Distribution of symbiotic bacteria in Steingelia gorodetskia (Steingeliidae). a Bacteria Sphingomonas (Sph) in the cytoplasm of cystocytes in the ovary of the last instar larva. Polyfusome (pf). TEM, scale bar = 2 μm. b Bacteria Sphingomonas (Sph) in the cytoplasm of trophocyte of the adult female. Mitochondrium (m); Trophocyte nucleus (tn). TEM, scale bar = 2 μm. c Bacteria Sphingomonas (Sph) in processes of trophocytes during the migration via the trophic core and the nutritive cord to the oocyte. TEM, scale bar = 2 μm. d Bacteria Sphingomonas (Sph) migrate through the trophic core to the oocyte. Mitochondrium (m). TEM, scale bar = 2 μm
The presence of bacteria (Wolbachia and Sodalis in O. urticae, Wolbachia in M. pini, Sphingomonas in S. gorodetskia) in the ovarioles of all the examined individuals indicates that these microorganisms are transovarially transmitted from one generation to the next. The bacteria to reach the oocyte migrate from trophocytes through the trophic core and nutritive cords (Figs. 2b, c and 3c, d) (for further details concerning ovary organization in Ortheziidae, Steingeliidae, and Matsucoccidae, see Szklarzewicz and Biliński 1995; Szklarzewicz 1997; Koteja et al. 2003; Szklarzewicz et al. 2014).
Discussion
Our molecular and detailed ultrastructural analyses showed that members of archaeococcoid scale insects: Orthezia urticae, Matsucoccus pini, and Steingelia gorodetskia, are host to bacterial associates. It should be stressed that M. pini and S. gorodetskia were regarded by Buchner (1966) as asymbiotic. Koteja et al. (2003), who were the first to find small, rod-shaped bacteria residing in all the cells constituting the ovarioles of S. gorodetskia, proposed several explanations of this discrepancy between their’s and Buchner’s observations, e.g., the bacteria were too small to be detected under light microscope, the observed bacteria occur in some populations of S. gorodetskia only, these microorganisms do not represent symbionts. Our results have resolved some of these questions; however, some of them remain still open. Both ultrastructural and molecular analyses of individuals collected in different years and different localities clearly indicated that there is no doubt that the bacteria Sphingomonas are present in all members of the species S. gorodetskia. Thus, it seems probable that due to the small size of these bacteria, Buchner was unable to observe them using paraffin technique. The function of these bacteria remains unclear; however, the lack of any symptoms of their destructive influence on growth and reproduction of S. gorodetskia (Koteja and Żak-Ogaza 1981; Koteja et al. 2003, this study) suggests the positive impact of Sphingomonas on these insects. This in turn leads us to the hypothesis that the bacterium Sphingomonas may function as the symbiont of S. gorodetskia. However, in order to determine the exact role of this bacterium, further genomic studies are required. It should be stressed that some features of this cohabitation, such as a lack of specialized bacteriocytes and distribution of the bacteria Sphingomonas in different internal organs of S. gorodetskia, indicate its very young condition. The symbionts of most scale insects are harbored in specialized bacteriocytes (Buchner 1965); however, in some species, e.g., Acanthococcus aceris and Gossyparia spuria (both belonging to family Eriococcidae), symbiotic bacteria Burkholderia do not occupy bacteriocytes, but are dispersed in fat body cells (Michalik et al. 2016).
The bacteria belonging to the genus Sphingomonas are free living microorganisms widely distributed in the environment (in fresh and sea water, in soil); however, some of them, such as, e.g., Sphingomonas paucimobilis, may be the cause of diseases in humans and animals (White et al. 1996; Takeuchi et al. 2001; Ryan and Adley 2010; Feng et al. 2014). To our knowledge, the bacterium Sphingomonas has not been reported as the intracellular symbiont of insects so far. Tang et al. (2010), who examined three populations of the brown planthopper, Nilaparvata lugens, detected alphaproteobacteria related to Sphingomonas in only two of them. Thus, the occurrence of the bacterium Sphingomonas in only some of the examined individuals indicated that it cannot be an obligate symbiont of Nilaparvata lugens.
Our molecular phylogenetic analyses have revealed that the bacterium Sphingomonas present in S. gorodetskia is closely related to Sphingomonas echinoides which, like other species of the genus Sphingomonas, commonly occurs in the soil (Takeuchi et al. 2001). This finding suggests that the bacteria residing in cells of all the individuals of S. gorodetskia are descendants of free living soil bacterium which has been acquired by an ancestor of these insects. It seems that the transition of bacterium Sphingomonas from free living to symbiotic status occurred according to the same evolutionary scenario as the acquisition of bacterium Burkholderia through eriococids A. aceris and G. spuria (Michalik et al. 2016). The bacteria of the genus Burkholderia, similarly to bacteria Sphingomonas, commonly occur in the soil and may be also plant, animal, and human pathogens (Stoyanova et al. 2007). It is worth mentioning that apart from the two species of eriococcids mentioned above, the symbiotic bacterium Burkholderia has also been detected in the crypts of the midgut of some stinkbugs (Kikuchi et al. 2005, 2007, 2011; Kikuchi and Fukatsu 2008; Itoh et al. 2014). Moreover, Kikuchi et al. (2005) and Itoh et al. (2014) have shown that the broad-headed stinkbug, Rhiptortus clavatus, and oriental chinch bug, Cavelerius saccarivorus, may transmit the bacterium Burkholderia between generations both vertically and horizontally (i.e., through the acquisition of these microorganisms by each generation directly from the environment). Kikuchi et al. (2007, 2011) have also revealed that symbiotic bacteria Burkholderia isolated from the midgut crypts of stinkbugs, in contrast to the bacteriocyte-associated symbionts of other hemipterans, grow on standard bacterial media. Thus, the observations mentioned above strongly support the view that symbioses of hemipterans and bacteria Burkholderia are at a much earlier stage than bacteriocyte symbioses in other insects. It should also be mentioned that the assumption that the bacterium Sphingomonas in S. gorodetskia is a descendant of the soil bacterium corresponds well with Koteja’s (1985) hypothesis, which maintains that an enormous diversity of symbionts associated with scale insects results from the permanent contact of ancestors of those insects with the soil bacteria in forest litter (i.e., the primary habitat of these insects). According to Koteja (1985), the groups of scale insects which have already diverged changed their feeding behavior from saprophagic into plant sap-sucking at a different time. Since the “new diet” required the support of symbionts, the particular groups of scale insects acquired different symbionts. It should be stressed that recent molecular analyses which show that the symbionts of scale insects belong to different bacterial taxa (von Dohlen et al. 2001; Thao et al. 2002; Gruwell et al. 2007, 2010, 2014; Kono et al. 2008; Matsuura et al. 2009; Ramirez-Puebla et al. 2010; Gatehouse et al. 2011; Dhami et al. 2012; Rosenblueth et al. 2012; Koga et al. 2013a; Rosas-Pérez et al. 2014; Michalik et al. 2016, 2018; Szabo et al. 2017; Szklarzewicz et al. 2018) strongly support this hypothesis.
Another argument supporting the assumption on the young condition of symbiosis in S. gorodetskia concerns the mode of the transmission of bacteria from mother to progeny. Both the results of earlier observations of Koteja et al. (2003) and present studies indicate that, in contrast to scale insects which are characterized by a long-lasting symbiosis with bacterial associates (i.e., representing “bacteriocyte symbiosis”), the females of S. gorodetskia have not yet developed a specialized mode of symbiont transmission. It is commonly known that different groups of scale insects are characterized by diverse methods of symbiont transmission (Buchner 1965; Szklarzewicz and Michalik 2017). In most scale insects, symbionts infect ovaries which contain oocytes during the advanced stage of vitellogenesis, e.g., in members of the family Pseudococcidae and Eriococcidae examined thus far, in Puto superbus (Putoidae), bacteria invade the anterior pole of the vitellogenic ovariole, whereas in Palaeococcus fuscipennis (Monophlebidae) and Porphyrophora polonica (Margarodidae) the posterior pole of the vitellogenic ovariole is infected (Buchner 1965, 1966; von Dohlen et al. 2001; Szklarzewicz et al. 2006, 2018; Michalik et al. 2016, 2018). In Marchalina hellenica (Marchalinidae) and Puto albicans (Putoidae), bacteria infect the undifferentiated germ cells (= cystocytes) constituting the larval ovaries (Szklarzewicz et al. 2010, 2013). Ultrastructural observations revealed that in S. gorodetskia none of the above mentioned modes of symbiont transmission occurs. It was observed that in the reproductive females of S. gorodetskia, bacteria Sphingomonas are dispersed in all the cells constituting the ovariole (in somatic cells, i.e., follicular cells and the cells of the ovariole sheath, as well as in germ cells, i.e., in oocytes and trophocytes). It seems probable that bacteria Sphingomonas, similarly to symbionts of M. hellenica and P. albicans (see above), infect the ovaries of the larvae before the differentiation of cystocytes into oocytes and trophocytes, however, apart from germ cells they also attack somatic cells. Since the symbiotic bacteria have to reach the oocyte (and, consequently, the next generation), they migrate from trophocytes to these cells via the trophic core and nutritive cord. Thus, ultrastructural observations indicate that in S. gorodetskia bacteria Sphingomonas are transovarially inherited; however, in not so specialized a way as in remaining scale insects. It is worth mentioning that bacteria Burkholderia, which represent the obligate symbionts of eriococcids A. aceris and G. spuria, in spite of their “less advanced” localization in fat body cells, are transmitted to the next generation in a mode typical for most hemipterans, i.e., through the infection of older oocytes (Michalik et al. 2016).
Both ultrastructural and molecular analyses have revealed that all the examined individuals of M. pini and O. urticae were colonized by numerous bacteria Wolbachia. The bacterium Wolbachia is widely distributed within insects, other arthropods, and nematodes (Werren 1997; Stouthamer et al. 1999; Werren et al. 2008). In most arthropods, bacterium Wolbachia is regarded as a “reproductive manipulator” or “reproductive parasite.” Since the bacterium Wolbachia is maternally inherited through the infection of oocytes, it developed several strategies of eliminating males, e.g., through the killing of male embryos, feminization of male embryos, cytoplasmic incompatibility in infected males and uninfected females, and the induction of parthenogenesis. So far, the nutritional, mutualistic relationship between Wolbachia and its host has been reported for filarial nematodes and bedbug Cimex lectularius only (Hosokawa et al. 2010; Slatko et al. 2010). Genomic analyses, as well as experiments with antibiotic treatment have shown that both in filarial nematodes and in C. lectularius bacterium Wolbachia plays an essential role for the proper growth and reproduction of the host: in filarial nematodes, the bacterium Wolbachia is responsible for heme biosynthesis (Slatko et al. 2010); in C. lectularius, this microorganism provides B vitamins (Hosokawa et al. 2010). Moreover, in C. lectularius, bacteria Wolbachia are harbored in specialized bacteriocytes, whereas in remaining insects these microorganisms are dispersed in different tissue. According to Nikoh et al. (2014), the situation observed in C. lectularius is an example of the evolutionary transition from facultative symbiosis to obligate nutritional mutualism.
There are only several reports on the occurrence of Wolbachia in scale insects (Duron et al. 2008; Matsuura et al. 2009; Vashishtha et al. 2011; Dhami et al. 2012; Szklarzewicz et al. 2018); however, the role of this bacterium in biology of examined scale insects remains still unknown. Our observations during the collection of M. pini and O. urticae in the field indicated that males are as numerous as the females. This, in turn, suggests that the bacterium Wolbachia does not negatively affect the number of males in the examined population of M. pini and O. urticae. Thus, to answer the question whether Wolbachia is beneficial for M. pini and O. urticae or if this bacterium represents only a guest microorganism, further studies are required.
It should be stressed that neither the use of ultrastructural nor molecular methods showed the presence of bacteria other than bacterium Wolbachia in the body of M. pini. The absence of other obligate symbionts in M. pini is probably related to the fact that these insects are parenchyma feeders (Siewniak 1976). Therefore, receiving nutritious food, scale insects of the Matsucoccus genus did not enter into symbiotic relationships with microorganisms supplementing their diet.
The presence of Sodalis-like bacteria in only some individuals of O. urticae suggests that this microorganism does not play a nutritional role in the biology of these insects. Taking into consideration the fact that Sodalis-like bacteria in numerous plant sap-sucking hemipterans represent “novel,” very expensive, obligate symbiont (Koga et al. 2013b; Koga and Moran 2014; Michalik et al. 2014; Vera-Ponce de León et al. 2016; Kobiałka et al. 2018a, b; Szklarzewicz et al. 2018), it is possible that the situation observed in O. urticae may be the beginning of the colonization of the scale insects of this species through Sodalis.
References
Baumann P (2005) Biology of bacteriocyte-associated endosymbionts of plant sap sucking insects. Annu Rev Microbiol 59:155–189
Ben-Dov Y (2005) A systematic catalogue of the scale insect family Margarodidae (Hemiptera: Coccoidea) of the world. Intercept Ltd, Wimborne
Ben-Dov Y (2012) The scale insects (Hemiptera: Coccoidea) of Israel—checklist, host plants, zoogeographical considerations and annotations on species. Isr J Entomol 41–42:21–48
Booth JM, Gullan PJ (2006) Synonymy of three pestiferous Matsucoccus scale insects (Hemiptera: Coccoidea: Matsucoccidae) based on morphological and molecular evidence. Proc Entomol Soc Wash 108:749–760
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Interscience Publishers, New York
Buchner P (1966) Endosymbiosestudien an Schildläusen. VIII. Die Symbiosen der Paleococcoidea. 1. Teil. Z Morphol Ökol Tiere 56:275–362
Cook LG, Gullan PJ, Trueman HE (2002) A preliminary phylogeny of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea) based on nuclear small-subunit ribosomal DNA. Mol Phylogenet Evol 25:43–52
Danzig EM (1980) Coccoids of the Far East USSR (Homoptera, Coccinea) with phylogenetic analysis of scale insects fauna of the world. 100th Nauka, Leningrad
Dhami MK, Turner AP, Deines P, Beggs JR, Taylor MW (2012) Ultrastructural and molecular characterization of a bacterial symbiosis in the ecologically important scale insect family Coelostomidiidae. FEMS Microbiol Ecol 81:537–546
Douglas AE (1989) Mycetocyte symbiosis in insects. Biol Rev 64:409–434
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:173–177
Duron O, Bouchon B, Boutin S, Bellamy L, Zhou L, Engelstädter J, Hurst GD (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27
Dziedzicka A (1977) Comparative investigations on the hind legs of scale insects (Coccinea). Scientific Publications Krakow Sup. Teachers’ College 20:1–105
Feng G-D, Yang S-Z, Wang Y-H, Zhang X-X, Zhao G-H, Deng M-R, Zhu H-H (2014) Description of a Gram-negative bacterium, Sphingomonas guangdongensis sp. nov. Int J Syst Evol Microbiol 64:1697–1702
Foldi I (2004) The Matsucoccidae in the Mediterranean basin with a world list of species (Hemiptera: Sternorrhyncha: Coccoidea). Ann Soc Entomol France 40:45–168
Foldi I (2005) Ground pearls: a generic revision of the Margarodidae sensu stricto. (Hemiptera: Sternorrhyncha: Coccoidea). Ann Soc Entomol France 41:81–125
Fukatsu T, Nikoh N (1998) Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl Environ Microbiol 64:3599–3606
Fukatsu T, Nikoh N (2000) Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl Environ Microbiol 66:643–650
Gatehouse LN, Sutherland P, Forgie SA, Kaji R, Christeller JT (2011) Molecular and histological characterization of primary (Betaproteobacteria) and secondary (Gammaproteobacteria) endosymbionts of three mealybug species. Appl Environ Microbiol 78:1187–1197
Gavrilov-Zimin IA (2018) Ontogenesis, morphology and higher classification of archaeococcids (Homoptera: Coccinea: Orthezioidea). Zoosyst Ross 2018:1–264
Gruwell ME, Morse GE, Normark BB (2007) Phylogenetic congruence of armored scale insects (Hemiptera: Diaspididae) and their primary endosymbionts from the phylum Bacteroidetes. Mol Phylogenet Evol 44:267–280
Gruwell ME, Hardy NB, Gullan PJ, Dittmar K (2010) Evolutionary relationships among primary endosymbionts of the mealybug subfamily Phenacoccinae (Hemiptera: Coccoidea: Pseudococcidae). Appl Environ Microbiol 76:7521–7525
Gruwell ME, Flarhety M, Dittmar K (2012) Distribution of the primary endosymbiont (Candidatus Uzinura diaspidicola) within host insects from the scale insect family Diaspididae. Insects 3:262–269
Gruwell ME, Duda Z, MacCready J (2014) Investigation of endosymbiotic bacteria associated with scale insects of the family Putoidae (Hemiptera: Coccoidea). Acta Zool Bulg 6:29–34
Gullan PJ, Cook LG (2007) Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Zootaxa 1668:413–425
Gullan PJ, Martin JH (2009) Sternorrhyncha (jumping plant-lice, whiteflies, aphids, and scale insects). In: Resh VH, Cardé RT (eds) Encyclopedia of insects. Elsevier, San Diego, pp 957–967
Hall TA (1999) BIOEDIT: an user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hardy NB, Gullan PJ, Hodgson CJ (2008) A subfamily-level classification of mealybugs (Hemiptera: Pseudococcidae) based on integrated molecular and morphological data. Syst Entomol 33:51–71
Hodgson CJ (2012) Comparison of the morphology of the adult males of the rhizoecine, phenacoccine and pseudococcine mealybugs (Hemiptera: Sternorrhyncha: Coccoidea), with the recognition of the family Rhizoecidae Williams. Zootaxa 3291:1–79
Hodgson CJ (2014) Phenacoleachia, Steingelia, Pityococcus and Puto—neococcoids or archaeococcoids? An intuitive phylogenetic discussion based on adult male characters. Acta Zool Bulg Supl 6:41–49
Hodgson CJ, Foldi I (2006) A review of the Margarodidae sensu Morrison (Hemiptera: Coccoidea) and some related taxa based on the morphology of adult males. Zootaxa 1263:1–250
Hodgson CJ, Hardy NB (2013) The phylogeny of the superfamily Coccoidea (Hemiptera: Sternorrhyncha) based on the morphology of extant and extinct macropterous males. Syst Entomol 38:794–804
Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. PNAS 107:769–774
Husnik FN, Nikoh R, Koga R, Ross L, Duncan RP, Fujie M, Tanaka M, Satoh N, Bachtrog D, Wilson ACC, von Dohlen CD, Fukatsu T, McCutcheon JP (2013) Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153:1567–1578
Itoh H, Aita M, Nagayama A, Meng X-Y, Kamagata Y, Navarro R, Hori T, Ohgiya S, Kikuchi Y (2014) Evidence of environmental and vertical transmission of Burkholderia symbionts in the oriental chinch bug, Cavelerius saccharivorus (Heteroptera: Blissidae). Appl Environ Microbiol 80:5974–5983
Kaydan M, Kozár F (2010) Soft scale insect (Hemiptera: Coccoidea) species of Eastern Anatolia of Turkey. Acta Phytopathologica et Entomologica Hungarica 45:195–221
Kikuchi Y, Fukatsu T (2008) Insect-bacterium mutualism without vertical transmission. In: Bourtzis K, Miller TA (eds) Insect symbiosis, CRC Press, vol 3, Boca Raton, pp 143–161
Kikuchi Y, Meng XY, Fukatsu T (2005) Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl Environ Microbiol 71:4035–4043
Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73:4308–4316
Kikuchi Y, Hosokawa T, Fukatsu T (2011) An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460
Kobiałka M, Michalik A, Szwedo J, Szklarzewicz T (2018a) Diversity of symbiotic microbiota in Deltocephalinae leafhoppers (Insecta, Hemiptera, Cicadellidae). Arthropod Struct Dev 47:268–278
Kobiałka M, Michalik A, Szklarzewicz T (2018b) An unusual symbiotic system in Elymana kozhevnikovi (Zachvatkin, 1938) and E. sulphurella (Zetterstedt, 1828) (Insecta, Hemiptera, Cicadellidae: Deltocephalinae). Folia Biol (Kraków) 66:13–24
Koga R, Moran NA (2014) Swapping symbionts in spittlebugs: evolutionary replacement of a reduced genome symbiont. ISME J 8:1237–1246
Koga R, Nikoh N, Matsuura Y, Meng X-Y, Fukatsu T (2013a) Mealybugs with distinct endosymbiotic systems living on the same host plant. FEMS Microbiol Ecol 83:93–100
Koga R, Bennett GM, Cryan JR, Moran NA (2013b) Evolutionary replacement of symbionts in an ancient and diverse insect lineage. Environ Microbiol 15:2073–2081
Kono M, Koga R, Shimada M, Fukatsu T (2008) Infection dynamics of coexisting β and γ-proteobacteria in the nested endosymbiotic system of mealybugs. Appl Environ Microbiol 74:4175–4184
Kosztarab MP (1996) Scale insects of northeastern North America. Identification, biology, and distribution. Virginia Museum of Natural History, Martinsburg
Kosztarab M, Kozár F (1988) Scale insects of Central Europe. Akademiai Kiado, Budapest
Koteja J (1974) Comparative studies on the labium in the Coccinea (Homoptera). Sci Pap Agric Univ Kraków 89:1–162
Koteja J (1985) Essay on the prehistory of the scale insects (Homoptera, Coccinea). Ann Zool 38:461–503
Koteja J (1996) Scale insects (Homoptera: Coccinea) a day after. In: Schaefer CW (ed) Studies on hemipteran phylogeny. Entomol Soc Am, Lanham, pp 65–88
Koteja J (2000) Scale insects (Homoptera, Coccinea) from Upper Cretaceous New Jersey amber. Studies on fossils in Amber, with particular reference to the cretaceous of New Jersey. Bol Mus Entomol Univ Valle Backhuys Publishers, Leiden
Koteja J, Żak-Ogaza B (1981) The life history of Steingelia gorodetskia Nassonov (Homoptera, Coccinea). Annal Zool 36:171–186
Koteja J, Pyka-Fosciak G, Vogelgesang M, Szklarzewicz T (2003) Structure of the ovary in Steingelia (Sternorrhyncha: Coccinea), and its phylogenetic implications. Arthropod Struct Dev 32:247–256
Kozár F, Miller D (2000) World revision of Ortheziola Šulc (Homoptera: Coccoidea: Ortheziidae) with descriptions of eleven new species. Syst Entomol 25:15–45
Kozár F, Konczné Benedicty Z, Fetykó K, Kiss B, Szita É (2013) An annotated update of the scale insect checklist of Hungary (Hemiptera, Coccoidea). Zookeys 309:49–66
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Matsuura Y, Koga R, Nikoh N, Meng XY, Hanada S, Fukatsu T (2009) Huge symbiotic organs in giant scale insects of the genus Drosicha (Coccoidea: Monophlebidae) harbor flavobacterial and enterobacterial endosymbionts. Zool Sci 26:448–456
McCutcheon JP, von Dohlen CD (2011) An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr Biol 21:1366–1372
Mech AM, Asaro C, Cram MM, Coyle DR, Gullan PJ, Cook LG, Gandhi KJK (2013) Matsucoccus macrocicatrices (Hemiptera: Matsucoccidae): first report, distribution, and association with symptomatic eastern white pine in the southeastern United States. J Econ Entomol 106:2391–2398
Michalik A, Jankowska W, Kot M, Gołas A, Szklarzewicz T (2014) Symbiosis in the green leafhopper, Cicadella viridis (Hemiptera, Cicadellidae). Association in statu nascendi? Arthropod Struct Dev 43:579–587
Michalik K, Szklarzewicz T, Kalandyk-Kołodziejczyk M, Jankowska W, Michalik A (2016) Bacteria belonging to the genus Burkholderia are obligatory symbionts of the eriococcids Acanthococcus aceris Signoret, 1875 and Gossyparia spuria (Modeer, 1778) (Insecta, Hemiptera, Coccoidea). Arthropod Struct Dev 45:265–272
Michalik A, Schulz F, Michalik K, Wascher F, Horn M, Szklarzewicz T (2018) Coexistence of novel gammaproteobacterial and Arsenophonus symbionts in the scale insect Greenisca brachypodii (Hemiptera, Coccomorpha: Eriococcidae). Environ Microbiol 20:1148–1157
Morales CF (1991) Margarodidae (Insecta: Hemiptera). Fauna of New Zealand, vol 21. DSIR Plant Protection, Auckland
Morrison H (1928) A classification of the higher groups and genera of the coccid family Margarodidae. US Dep Agric Tech Bull 52:1–239
Nikoh N, Hosokawa T, Moriyama M, Oshima, Hattori M, Fukatsu T (2014) Evolutionary origin of insect-Wolbachia nutritional mutualism. PNAS 111:10257–10262
Niżnik S, Szklarzewicz T (2007) Structure and development of hermaphroditic gonad in Icerya purchasi (Insecta, Hemiptera, Coccinea: Monophlebidae). Zool Polon 52:71–90
Podsiadło E, Michalik K, Michalik A, Szklarzewicz T (2018) Yeast-like microorganisms in the scale insect Kermes quercus (Insecta, Hemiptera, Coccomorpha: Kermesidae). Newly acquired symbionts? Arthropod Struct Dev 47:56–63
Ramirez-Puebla ST, Rosenblueth M, Chavez-Moreno CK, de Catanho Pereira Lyra MC, Tecante A, Martinez-Romero A (2010) Molecular phylogeny of the genus Dactylopius (Hemiptera: Dactylopiidae) and identification of the symbiotic bacteria. Environ Entomol 39:1178–1183
Rosas-Pérez T, Rosenblueth M, Rincón-Rosales R, Mora J, Martínez-Romero E (2014) Genome sequence of “Candidatus Walczuchella monophlebidarum” the flavobacterial endosymbiont of Llaveia axin axin (Hemiptera: Coccoidea: Monophlebidae). Genome Biol Evol 6:714–726
Rosenblueth M, Sayavedra L, Sámano-Sánchez H, Roth A, Martínez-Romero E (2012) Evolutionary relationships of flavobacterial and enterobacterial endosymbionts with their scale insect hosts (Hemiptera: Coccoidea). J Evol Biol 25:2357–2368
Rosenblueth M, Martínez-Romero J, Ramírez-Puebla ST, Vera-Ponce de León A, Rosas-Pérez T, Bustamante-Brito R, Rincón-Rosales R, Martínez-Romero E (2018) Endosymbiotic microorganisms of scale insects. Rev Espec Cienc Quím-Biol 21:53–69
Ryan MP, Adley CC (2010) Sphingomonas paucimobilis: a persistent Gram-negative nosocomial infectious organism. J Hosp Infect 75:153–157
Sabree ZL, Huang CY, Okusu A, Moran NA, Normark BB (2013) The nutrient supplying capabilities of Uzinura, an endosymbiont of armoured scale insects. Environ Microbiol 15:1988–1999
Siewniak M (1976) Zur Morphologie und Bionomie der Kiefernborkenschildlaus, Matsucoccus pini (Green) (Hom., Coccoidea: Margarodidae). Z Angew Entomol 81:337–362
Slatko BE, Taylor MJ, Foster JM (2010) The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51:55–65
Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Stoyanova M, Pavlina I, Moncheva P, Bogatzevska N (2007) Biodiversity and incidence of Burkholderia species. Biotechnol Biotechnol Equip 21:306–310
Szabo G, Schulz F, Toenshoff ER, Volland J-M, Omri M, Finkel OM, Belkin S, Horn M (2017) Convergent patterns in the evolution of mealybug symbioses involving different intrabacterial symbionts. ISME J 11:715–726
Szklarzewicz T (1997) Structure and development of the telotrophic ovariole in ensign scale insects (Hemiptera, Coccomorpha: Ortheziidae). Tissue Cell 29:31–38
Szklarzewicz T, Biliński SM (1995) Structure of ovaries in ensign scale insects, the most primitive representatives of Coccomorpha (Insecta, Hemiptera). J Morphol 224:23–29
Szklarzewicz T, Michalik A (2017) Transovarial transmission of symbionts in insects. In: Kloc M (ed) Molecular mechanisms of cell differentiation in gonad development. Springer, Cham, pp 46–67
Szklarzewicz T, Kędra K, Niżnik S (2006) Ultrastructure and transovarial transmission of endosymbiotic microorganisms in Palaeococcus fuscipennis (Burmeister) (Insecta, Hemiptera, Coccinea: Monophlebidae). Folia Biol (Kraków) 54:70–74
Szklarzewicz T, Michalik A, Czaja A, Szydłowska S (2010) Germ cell cluster formation and ovariole structure in Puto albicans and Crypticerya morrilli (Insecta, Hemiptera, Coccinea). Phylogenetic implications. Eur J Entomol 107:589–596
Szklarzewicz T, Kalandyk-Kołodziejczyk M, Kot M, Michalik A (2013) Ovary structure and transovarial transmission of endosymbiotic microorganisms in Marchalina hellenica (Insecta, Hemiptera, Coccomorpha: Marchalinidae). Acta Zool Stockholm 94:184–192
Szklarzewicz T, Michalik A, Kalandyk-Kołodziejczyk M, Kobiałka M, Simon E (2014) Ovary of Matsucoccus pini (Insecta, Hemiptera, Coccinea: Matsucoccidae): morphology, ultrastructure and phylogenetic implications. Microsc Res Tech 77:327–334
Szklarzewicz T, Kalandyk-Kołodziejczyk M, Michalik K, Jankowska W, Michalik A (2018) Symbiotic microorganisms in Puto superbus (Leonardi, 1907) (Insecta, Hemiptera, Coccomorpha: Putoidae). Protoplasma 255:129–138
Takeuchi M, Hamana K, Hiraishi A (2001) Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int J Syst Evol Microbiol 51:1405–1417
Tang M, Lv L, Jing S, Zhu L, He G (2010) Bacterial symbionts of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl Environ Microbiol 76:1740–1745
Thao ML, Gullan PJ, Baumann P (2002) Secondary (γ-proteobacteria) endosymbionts infect the primary (β-proteobacteria) endosymbionts of mealybugs multiple times and coevolve with their host. Appl Environ Microbiol 68:3190–3197
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tremblay E (1977) Advances in endosymbiont studies in Coccoidea. Virginia Polytech Inst State Univ Res Div Bull 127:23–33
Vashishtha A, Sharama K, Lakhanpaul S (2011) Co-existence, phylogeny and putative role of Wolbachia and yeast-like symbiont (YLS) in Kerria lacca (Kerr). Curr Microbiol 63:206–212
Vea I, Grimaldi D (2012) Phylogeny of ensign scale insects (Hemiptera: Coccoidea: Ortheziidae) based on the morphology of recent and fossil females. Syst Entomol 37:758–783
Vera-Ponce de León A, Sanchez-Flores A, Rosenblueth M, Martínez-Romero E (2016) Fungal community associated with Dactylopius (Hemiptera: Coccoidea: Dactylopiidae) and its role in uric acid metabolism. Front Microbiol 7:954
von Dohlen CD, Kohler S, Alsop ST, McManus WR (2001) Mealybug β-proteobacterial endosymbionts contain γ-proteobacterial symbionts. Nature 412:433–435
Walczuch A (1932) Studien an Coccidensymbionten. Z Morphol Ökol Tiere 25:623–729
Wang X, Xie YP, Zhang YF, Liu WM, Wu J (2016) The structure and morphogenic changes of antennae of Matsucoccus matsumurae (Hemiptera: Coccoidea: Matsucoccidae) in different instars. Arthropod Struct Dev 45:281–293
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609
Werren JH, Baldo L, Clark SE (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
White DC, Suttont SD, Ringelberg DB (1996) The genus Sphingomonas: physiology and ecology. Curr Opin Biotechnol 7:301–306
Williams DJ, Hodgson CJ (2014) The case for using the infraorder Coccomorpha above the superfamily Coccoidea for the scale insects (Hemiptera: Sternorrhyncha). Zootaxa 3869:348–350
Williams DJ, Watson GW (1990) The scale insects of the tropical South Pacific region. Pt. 3: the soft scales (Coccidae) and other families. CAB International, Wallingford
Williams DJ, Gullan PJ, Miller DR, Matile-Ferrero D, Han SI (2011) A study of the scale insect genera Puto Signoret (Hemiptera: Sternorrhyncha: Coccoidea: Putoidae) and Ceroputo Sulc (Pseudococcidae) with a comparison to Phenacoccus Cockerell (Pseudococcidae). Zootaxa 2802:1–22
Zhou W, Rousset F, O'Neil S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515
Acknowledgements
We would like to express our gratitude to Msc Ada Jankowska (Jagiellonian University, Kraków) for their skilled technical assistance and Dr. Ewa Simon (University of Silesia, Katowice) for collection and identification of specimens. Ultrastructural observations have been carried out using the JEM 100 SX EM and Jeol JEM 2100 transmission electron microscopes in the Laboratory of Microscopy, Department of Cell Biology and Imaging, Institute of Zoology and Biomedical Research, Jagiellonian University.
Funding
This work was supported by the Iuventus Plus V research grant IP2015050374 in the years 2016–2019 from the Ministry of Science and Higher Education to Anna Michalik.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling Editor: Pavel Dráber
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
a Young adult female of Orthezia urticae (Ortheziidae) (photographed by Katarzyna Michalik). b Female of Steingelia gorodetskia (Steingeliidae) (photographed by Katarzyna Michalik). c Female of Matsucoccus pini (Matsucoccidae) (photographed by Marzena Zmarzły). Stereomicroscope, scale bar = 1 mm (PNG 5363 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Michalik, K., Szklarzewicz, T., Kalandyk-Kołodziejczyk, M. et al. Bacterial associates of Orthezia urticae, Matsucoccus pini, and Steingelia gorodetskia - scale insects of archaeoccoid families Ortheziidae, Matsucoccidae, and Steingeliidae (Hemiptera, Coccomorpha). Protoplasma 256, 1205–1215 (2019). https://doi.org/10.1007/s00709-019-01377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01377-z