Abstract
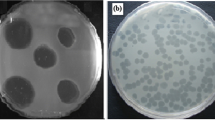
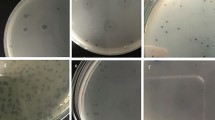
Proteus mirabilis is responsible for a wide range of infections that affect the urinary tract, the respiratory tract, burns, wounds and the feet of individuals with diabetes. They are highly resistant to antimicrobial agents, and new therapeutic options are therefore needed to combat this pathogen. The use of bacteriophages is one option that may be useful in treating multidrug-resistant (MDR) Proteus mirabilis infections, especially biofilm-based infections. The aim of this study was to control biofilms formed by MDR Proteus mirabilis using bacteriophages. Proteus mirabilis isolates were identified based on biochemical tests, and their resistance profiles were determined by the disk diffusion method. The biofilm-forming capacity of the isolates was assessed by the spectrophotometric method. Bacteriophages attacking Proteus mirabilis were isolated from sewage. The effect of phage on biofilm formation was investigated by the viable count method. A high rate of drug resistance was found (87.2%). Strong biofilm formation was observed in 80.5% of isolates, while moderate production was found in 19.5%. Five bacteriophages were isolated from sewage and were tested for their ability to eliminate biofilms. Significant disruption of pre-formed biofilms was observed that reached up to 99.9% decrease in the number of viable cells. The use of bacteriophages is considered a promising strategy against the biofilm infections caused by MDR Proteus mirabilis isolates.



Similar content being viewed by others
References
Abbas HA, Gad AI (2014) Eradication of biofilms formed by bacteria isolated from diabetic foot infections by potential antibiofilm agents alone and in combination with ciprofloxacin. Afr J Microbiol Res 8:3882–3892
Abbas HA, El-Saysed MA, Ganiny AM, Fattah AA (2018) Antimicrobial resistance patterns of Proteus mirabilis isolates from urinary tract, burn wound and diabetic foot infections. Res J Pharm Tech 11:249–252
Adeolu M, Alnajar S, Naushad S, Gupta RS (2016) Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int J Syst Evol Microbiol 66:5575–5599
Afriani R, Rusmana I, Budiarti S (2014) Characterization of Proteus mirabilis lytic phage from Situ Letik River Bogor Indonesia. Int J Innovat Res Sci Eng 2:2347–3207
Akova M (2016) Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 7:252–266
Azeredo J, Sutherland IW (2008) The use of phages for the removal of infectious biofilms. Curr Pharm Biotechnol 9:261–266
Carey-Smith GV, Billington C, Cornelius AJ, Hudson JA, Heinemann JA (2006) Isolation and characterization of bacteriophages infecting Salmonella spp. FEMS Microbiol Lett 258:182–186
Carlton RM (1999) Phage therapy: past history and future prospects. Arch Immunol Ther Exp (Warsz) 47:267–274
Carson L, Gorman SP, Gilmore BF (2010) The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol 59:447–455
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A (1999) The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776
Cerveny KE, DePaola A, Duckworth DH, Gulig PA (2002) Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect Immun 70:6251–6262
Chan BK, Abedon ST, Loc-Carrillo C (2013) Phage cocktails and the future of phage therapy. Fut Microbiol 8:769–783
Chen C-Y, Chen Y-H, Lu P-L, Lin W-R, Chen T-C, Lin C-Y (2012) Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. J Microbiol Immunol Infect 45:228–236
Clark JR, March JB (2006) Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials. Trends Biotechnol 24:212–218
CLSI-Clinical and Laboratory Standards Institute (2016) Performance standards for antimicrobial susceptibility testing, CLSI document M100-S-26. CLSI-Clinical and Laboratory Standards Institute, Wayne
Coker C, Poore CA, Li X, Mobley HL (2000) Pathogenesis of Proteus mirabilis urinary tract infection. Microb Infect 2:1497–1505
Cornelissen A, Ceyssens P-J, T’syen J, Van Praet H, Noben J-P, Shaburova OV, Krylov VN, Volckaert G, Lavigne R (2011) The T7-related Pseudomonas putida phage φ15 displays virionassociated biofilm degradation properties. PLoS One 6:e18597
Danis-Wlodarczyk K, Olszak T, Arabski M, Wasik S, Majkowska-Skrobek G, Augustyniak D, Gula G, Briers Y, Jang HB, Vandenheuvel D, Duda KA, Lavigne R, Drulis-Kawa Z (2015) Characterization of the newly isolated lytic bacteriophages KTN6 and KT28 and their efficacy against Pseudomonas aeruginosa biofilm. PLoS One 10:e0127603
Endimiani A, Luzzaro F, Brigante G, Perilli M, Lombardi G, Amicosante G, Rossolini GM, Toniolo A (2005) Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob Agents Chemother 49:2598–2605
Fard RMN, Barton MD, Heuzenroeder MW (2010) Novel Bacteriophages in Enterococcus spp. Curr Microbiol 60:400–406
Ghannad MS, Mohammadi A (2012) Bacteriophage: time to re-evaluate the potential of phage therapy as a promising agent to control multidrug-resistant bacteria. Iran J Basic Med Sci 15:693–701
Goodridge LD (2010) Designing phage therapeutics. Curr Pharm Biotechnol 11:15–27
Gurnev PA, Oppenheim AB, Winterhalter M, Bezrukov SM (2006) Docking of a single phage lambda to its membrane receptor maltoporin as a time-resolved event. J Mol Biol 359:1447–1455
Gutiérrez D, Vandenheuvel D, Martínez B, Rodríguez A, Lavigne R, García P (2015) Two phages, phiIPLA-RODI and phiIPLA-C1C, lyse mono-and dual-species Staphylococcal biofilms. Appl Environ Microbiol 81:3336–3348
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11:1034–1043
Harper D, Enright M (2011) Bacteriophages for the treatment of Pseudomonas aeruginosa infections. J Appl Microbiol 111:1–7
Harrison JJ, Turner RJ, Joo DA, Stan MA, Chan CS, Allan ND, Vrionis HA, Olson ME, Ceri H (2008) Copper and quaternary ammonium cations exert synergistic bactericidal and anti-biofilm activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:2870–2881
Ho K (2001) Bacteriophage therapy for bacterial infections. Rekindling a memory from the pre-antibiotics era. Perspect Biol Med 44:1–16
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Jacobsen SM, Shirtliff ME (2011) Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2:460–465
Jun JW, Kim JH, Shin SP, Han JE, Chai JY, Park SC (2013) Characterization and complete genome sequence of the Shigella bacteriophage pSf-1. Res Microbiol 164:979–986
Karaca B, Akcelik N, Akcelik M (2015) Effects of P22 bacteriophage on Salmonella enterica subsp. enterica serovar Typhimurium DMC4 strain biofilm formation and eradication. Arch Biol Sci 67:1361–1367
Karumidze N, Kusradze I, Rigvava S, Goderdzishvili M, Rajakumar K, Alavidze Z (2013) Isolation and characterisation of lytic bacteriophages of Klebsiella pneumoniae and Klebsiella oxytoca. Curr Microbiol 66:251–258
Koneman E, Winn WC, Allen S, Janda W, Procop G, Schreckenberger P, Woods G (2006) Koneman’s color atlas and textbook of diagnostic microbiology, 6th edn. Lippincott Williams & Wilkins, Pennsylvania
Koskella B, Meaden S (2013) Understanding bacteriophage specificity in natural microbial communities. Viruses 5:806–823
Krylov VN (2001) Phagotherapy in terms of bacteriophage genetics: hopes, perspectives, safety, limitations. Genetika 37:869–887
Kutateladze M, Adamia R (2010) Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol 28:591–595
Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11:69–86
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327
Lehman SM, Donlan RM (2015) Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother 59:1127–1137
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Lewis K (2010) Persister cells. Annu Rev Microbiol 64:357–372
Liu M, Deora R, Doulatov SR, Gingery M, Eiserling FA, Preston A, Maskell DJ, Simons RW, Cotter PA, Parkhill J, Miller JF (2002) Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295:2091–2094
Loc-Carrillo C, Abedon ST (2011) Pros and cons of phage therapy. Bacteriophage 1:111–114
Lynch AS, Robertson GT (2008) Bacterial and fungal biofilm infections. Annu Rev Med 59:415–428
Melo LD, Veiga P, Cerca N, Kropinski AM, Almeida C, Azeredo J, Sillankorva S (2016) Development of a phage cocktail to control Proteus mirabilis catheter-associated urinary tract infections. Front Microbiol 7:1024–1035
Modi SR, Collins JJ, Relman DA (2014) Antibiotics and the gut microbiota. J Clin Invest 124:4212–4218
Morozova V, Kozlova Y, Shedko E, Kurilshikov A, Babkin I, Tupikin A, Yunusova A, Chernonosov A, Baykov I, Capital Kabul CI, Kabilov M, Ryabchikova E, Vlassov V, Tikunova N (2016) Lytic bacteriophage PM16 specific for Proteus mirabilis: a novel member of the genus Phikmvvirus. Arch Virol 161:2457–2472
Morozova V, Kozlova Y, Shedko E, Babkin I, Kurilshikov A, Bokovaya O, Bardashova A, Yunusova A, Tikunov A, Tupikin A, Ushakova T, Ryabchikova E, Tikunova N (2018) Isolation and characterization of a group of new Proteus bacteriophages. Arch Virol 163:2189–2197
Nzakizwanayo J, Hanin A, Alves DR, McCutcheon B, Dedi C, Salvage J, Knox K, Stewart B, Metcalfe A, Clark J (2015) Bacteriophage can prevent encrustation and blockage of urinary catheters by Proteus mirabilis. Antimicrob Agents Chemother 60:1530–1536
Parasion S, Kwiatek M, Gryko R, Mizak L, Malm A (2014) Bacteriophages as an alternative strategy for fighting biofilm development. Pol J Microbiol 63:137–145
Perim MC, Borges Jda C, Celeste SR, Orsolin Ede F, Mendes RR, Mendes GO, Ferreira RL, Carreiro SC, Pranchevicius MC (2015) Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Br Med Trop 48:546–554
Projan S (2004) Phage-inspired antibiotics? Nat Biotechnol 22:167–168
Różalski A, Sidorczyk Z, Kotelko K (1997) Potential virulence factors of Proteus bacilli. Microbiol Mol Biol Rev 61:65–89
Rydman PS, Bamford DH (2002) The lytic enzyme of bacteriophage PRD1 is associated with the viral membrane. J Bacteriol 184:104–110
Sekhar S, Vyas N, Unnikrishnan M, Rodrigues G, Mukhopadhyay C (2014) Antimicrobial susceptibility pattern in diabetic foot ulcer: a pilot study. Ann Med Health Sci Res 4:742–745
Shanmugam P, Jeya M, Susan SL (2013) The bacteriology of diabetic foot ulcers, with a special reference to multidrug resistant strains. J Clin Diagn Res 7:441–445
Shapiro OH, Kushmaro A (2011) Bacteriophage ecology in environmental biotechnology processes. Curr Opin Biotechnol 22:449–455
Skurnik M, Strauch E (2006) Phage therapy: facts and fiction. Int J Med Microbiol 296:5–14
Stepanović S, Vuković D, Hola V, Bonaventura GD, Djukić S, Ćirković I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Acta Pathol Microbiol Immunol Scand B Microbiol 115:891–899
Stewart PS (2002) Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 292:107–113
Stickler DJ (2014) Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med 276:120–129
Sutherland IW, Hughes KA, Skillman LC, Tait K (2004) The interaction of phage and biofilms. FEMS Microbiol Lett 232:1–6
Thompson R (2018) The isolation and characterisation of Proteus mirabilis bacteriophages and their effect on the colonisation and blockage of urinary catheters. (PhD thesis), Faculty of Health and Applied Sciences, University of the West of England, Bristol
Trachoo N (2004) Biofilm removal technique using sands as a research tool for accessing microbial attachment on surface. Songklanakarin J Sci Technol 26:109–115
Yah S, Enabulele I, Yusuf E, Eghafona N (2006) Emerging quinolones resistant transfer genes among gram-negative bacteria isolated from faeces of HIV/AIDS patient attending some clinic and hospital in the city of Benin, Edo State, Nigeria. Online J Health Allied Sci 5:61–91
Yazdi M, Bouzari M, Ghaemi EA (2018) Isolation and characterization of a lytic bacteriophage (vB_PmiS-TH) and its application in combination with ampicillin against planktonic and biofilm forms of Proteus mirabilis isolated from urinary tract infection. J Mol Microbiol Biotechnol 28:37–46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: T. K. Frey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomaa, S., Serry, F., Abdellatif, H. et al. Elimination of multidrug-resistant Proteus mirabilis biofilms using bacteriophages. Arch Virol 164, 2265–2275 (2019). https://doi.org/10.1007/s00705-019-04305-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04305-x