Abstract
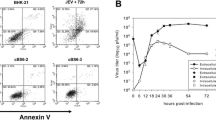
Enterovirus 71 (EV71) is one of the major causative agents of hand, foot and mouth disease (HFMD), which mainly occurs in children. Children with EV71 infection can develop severe neurological diseases. Heat shock protein 78 (HSP78) facilitates proper protein folding during viral propagation and is induced during virus infection. Nevertheless, the role that HSP78 plays during EV71 infection is still unclear. In this study, recombinant HSP78 protein was expressed in a prokaryotic expression system and used for exploring the interaction between HSP78 and EV71 propagation. Detection using a mouse immune anti-HSP78 serum in ELISA and western blot demonstrated that the recombinant HSP78 antigen is highly immunogenic. Furthermore, the recombinant HSP78 protein was able to bind to EV71 VP1 and intensified the cytopathic effect and viral propagation during EV71 infection, while the immune serum had a counteractive effect. However, knockdown of the HSP78 gene in Vero cells before EV71 infection did not result in a reduced virus titer. In addition, HSP78 on the cell surface was upregulated in human neuroblastoma cells (SK-N-SH) infected with EV71.









Similar content being viewed by others
References
Ye X, Fan C, Ku Z, Zuo T, Kong L, Zhang C, Shi J, Liu Q, Chen T, Zhang Y et al (2016) Structural basis for recognition of human enterovirus 71 by a bivalent broadly neutralizing monoclonal antibody. PLoS Pathog 12(3):e1005454
Caine EA, Moncla LH, Ronderos MD, Friedrich TC, Osorio JE (2016) A single mutation in the VP1 of enterovirus 71 is responsible for increased virulence and neurotropism in adult interferon-deficient mice. J Virol 90(19):8592–8604
Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y et al (2014) Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 14(4):308–318
Kim HJ, Hyeon JY, Hwang S, Lee YP, Lee SW, Yoo JS, Kang B, Ahn JB, Jeong YS, Lee JW (2016) Epidemiology and virologic investigation of human enterovirus 71 infection in the Republic of Korea from 2007 to 2012: a nationwide cross-sectional study. BMC Infect Dis 16(1):425
Lai CC, Jiang DS, Wu HM, Chen HH (2016) A dynamic model for the outbreaks of hand, foot, and mouth disease in Taiwan. Epidemiol Infect 144(7):1500–1511
Lim CT, Jiang L, Ma S, James L, Ang LW (2016) Basic reproduction number of coxsackievirus type A6 and A16 and enterovirus 71: estimates from outbreaks of hand, foot and mouth disease in Singapore, a tropical city-state. Epidemiol Infect 144(5):1028–1034
Huang Y, Zhou Y, Lu H, Yang H, Feng Q, Dai Y, Chen L, Yu S, Yao X, Zhang H et al (2015) Characterization of severe hand, foot, and mouth disease in Shenzhen, China, 2009–2013. J Med Virol 87(9):1471–1479
Yang B, Lau EH, Wu P, Cowling BJ (2016) Transmission of hand, foot and mouth disease and its potential driving factors in Hong Kong. Sci Rep 6:27500
Onozuka D, Hashizume M (2011) The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ 410–411:119–125
Ling BP, Jalilian FA, Harmal NS, Yubbu P, Sekawi Z (2014) Detection and characterization of viruses causing hand, foot and mouth disease from children in Seri Kembangan, Malaysia. Trop Biomed 31(4):654–662
Donato C, le Hoi T, Hoa NT, Hoa TM, Van Duyet L, Dieu Ngan TT, Van Kinh N, Vu Trung N, Vijaykrishna D (2012) Genetic characterization of enterovirus 71 strains circulating in Vietnam in 2012. Virology 495:1–9
Ham H, Woolery AR, Tracy C, Stenesen D, Kramer H, Orth K (2014) Unfolded protein response-regulated Drosophila Fic (dFic) protein reversibly AMPylates BiP chaperone during endoplasmic reticulum homeostasis. J Biol Chem 289(52):36059–36069
Reid SP, Shurtleff AC, Costantino JA, Tritsch SR, Retterer C, Spurgers KB, Bavari S (2014) HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 109:171–174
Gonzalez-Gronow M, Gomez CF, de Ridder GG, Ray R, Pizzo SV (2014) Binding of tissue-type plasminogen activator to the glucose-regulated protein 78 (GRP78) modulates plasminogen activation and promotes human neuroblastoma cell proliferation in vitro. J Biol Chem 289(36):25166–25176
Roberts JL, Tavallai M, Nourbakhsh A, Fidanza A, Cruz-Luna T, Smith E, Siembida P, Plamondon P, Cycon KA, Doern CD et al (2015) GRP78/Dna K is a target for Nexavar/Stivarga/Votrient in the treatment of human malignancies, viral infections and bacterial diseases. J Cell Physiol 230(10):2552–2578
Moreno JA, Tiffany-Castiglioni E (2015) The chaperone Grp78 in protein folding disorders of the nervous system. Neurochem Res 40(2):329–335
Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS (2014) GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene 33(42):4997–5005
Triantafilou K, Fradelizi D, Wilson K, Triantafilou M (2002) GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol 76(2):633–643
Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV (2009) GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal 11(9):2299–2306
Wu YP, Chang CM, Hung CY, Tsai MC, Schuyler SC, Wang RY (2011) Japanese encephalitis virus co-opts the ER-stress response protein GRP78 for viral infectivity. Virol J 8:128
Wang L, Mei M, Qin A, Ye J, Qian K, Shao H (2016) Membrane-associated GRP78 helps subgroup J avian leucosis virus enter cells. Vet Res 47(1):92
Alayli F, Scholle F (2016) Dengue virus NS1 enhances viral replication and pro-inflammatory cytokine production in human dendritic cells. Virology 496:227–236
Shi-Chen OuD, Lee SB, Chu CS, Chang LH, Chung BC, Juan LJ (2011) Transcriptional activation of endoplasmic reticulum chaperone GRP78 by HCMV IE1-72 protein. Cell Res 21(4):642–653
Wei D, Li NL, Zeng Y, Liu B, Kumthip K, Wang TT, Huo D, Ingels JF, Lu L, Shang J et al (2016) The molecular chaperone GRP78 contributes to Toll-like receptor 3-mediated innate immune response to hepatitis C virus in hepatocytes. J Biol Chem 291(23):12294–12309
Medigeshi GR, Lancaster AM, Hirsch AJ, Briese T, Lipkin WI, Defilippis V, Fruh K, Mason PW, Nikolich-Zugich J, Nelson JA (2007) West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol 81(20):10849–10860
Jheng JR, Wang SC, Jheng CR, Horng JT (2016) Enterovirus 71 induces dsRNA/PKR-dependent cytoplasmic redistribution of GRP78/BiP to promote viral replication. Emerg Microbes Infect 5:e23
Niu R, Chen X (2016) Full-length cDNA, prokaryotic expression, and antimicrobial activity of UuHb-F-I from Urechis unicinctus. Biomed Res Int 2016:5683026
Illiano E, Demurtas OC, Massa S, Di Bonito P, Consalvi V, Chiaraluce R, Zanotto C, De Giuli Morghen C, Radaelli A, Venuti A et al (2016) Production of functional, stable, unmutated recombinant human papillomavirus E6 oncoprotein: implications for HPV-tumor diagnosis and therapy. J Transl Med 14(1):224
Przybylski M, Borysowski J, Jakubowska-Zahorska R, Weber-Dabrowska B, Gorski A (2015) T4 bacteriophage-mediated inhibition of adsorption and replication of human adenovirus in vitro. Future Microbiol 10(4):453–460
Tang WF, Huang RT, Chien KY, Huang JY, Lau KS, Jheng JR, Chiu CH, Wu TY, Chen CY, Horng JT (2016) Host microRNA miR-197 plays a negative regulatory role in the enterovirus 71 infectious cycle by targeting the RAN protein. J Virol 90(3):1424–1438
White J, Hughes-Stamm S, Gangitano D (2015) Development and validation of a rapid PCR method for the PowerPlex(R) 16 HS system for forensic DNA identification. Int J Legal Med 129(4):715–723
Kono N, Nakamura H, Ito Y, Tomita M, Arakawa K (2016) Evaluation of the impact of RNA preservation methods of spiders for de novo transcriptome assembly. Mol Ecol Resour 16(3):662–672
Verma SK, Batra L, Tuteja U (2016) A recombinant trivalent fusion protein F1-LcrV-HSP70(II) augments humoral and cellular immune responses and imparts full protection against Yersinia pestis. Front Microbiol 7(1053):1–10
Chervyakova OV, Zaitsev VL, Iskakov BK, Tailakova ET, Strochkov VM, Sultankulova KT, Sandybayev NT, Stanbekova GE, Beisenov DK, Abduraimov YO et al (2016) Recombinant sheep pox virus proteins elicit neutralizing antibodies. Viruses 8(159):1–13
Wang J, Zhong M, Liu B, Sha L, Lun Y, Zhang W, Li X, Wang X, Cao J, Ning A et al (2015) Expression and functional analysis of novel molecule—latcripin-13 domain from Lentinula edodes C91-3 produced in prokaryotic expression system. Gene 555(2):469–475
Li Y, Lin Z, Zhao M, Xu T, Wang C, Hua L, Wang H, Xia H, Zhu B (2016) Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl Mater Interfaces 8(37):24385–24393
Lin H, Chen Y, Huang Q, Guo X, Liu P, Liu W, Zhang C, Cao H, Hu G (2016) Prokaryotic expression of the chicken xanthine oxidase (XOD) subunit and its localization in liver and kidney. Int J Biol Macromol 87:341–347
Li Y, Lin Z, Zhao M, Xu T, Wang C, Xia H, Wang H, Zhu B (2016) Multifunctional selenium nanoparticles as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. Int J Nanomed 11:3065–3076
Wang L, Yang S, Zhao K, Han L (2015) Expression profiles of the heat shock protein 70 gene in response to heat stress in Agrotis c-nigrum (Lepidoptera: Noctuidae). J Insect Sci 15:169
Ganguly A, Malabadi RB, Das D, Suresh MR, Sunwoo HH (2013) Enhanced prokaryotic expression of dengue virus envelope protein. J Pharm Pharm Sci 16(4):609–621
Fu X, Li N, Lin Q, Guo H, Liu L, Huang Z, Wu S (2015) Early protein ORF086 is an effective vaccine candidate for infectious spleen and kidney necrosis virus in mandarin fish Siniperca chuatsi. Fish Shellfish Immunol 46(2):200–205
Ali A, Nisar M, Idrees M, Rafique S, Iqbal M (2015) Expression of Hepatitis C Virus Core and E2 antigenic recombinant proteins and their use for development of diagnostic assays. Int J Infect Dis 34:84–89
Zhang Y, Wang Z, Zhan Y, Gong Q, Yu W, Deng Z, Wang A, Yang Y, Wang N (2016) Generation of E. coli-derived virus-like particles of porcine circovirus type 2 and their use in an indirect IgG enzyme-linked immunosorbent assay. Arch Virol 161(6):1485–1491
Yu HC, Lai PH, Lai NS, Huang HB, Koo M, Lu MC (2016) Increased serum levels of anti-carbamylated 78-kDa glucose-regulated protein antibody in patients with rheumatoid arthritis. Int J Mol Sci 17(9)
Li L, Zheng Q, Zhang Y, Li P, Fu Y, Hou J, Xiao X (2016) Antiviral activity of recombinant porcine surfactant protein A against porcine reproductive and respiratory syndrome virus in vitro. Arch Virol 161(7):1883–1890
Su PY, Wang YF, Huang SW, Lo YC, Wang YH, Wu SR, Shieh DB, Chen SH, Wang JR, Lai MD et al (2015) Cell surface nucleolin facilitates enterovirus 71 binding and infection. J Virol 89(8):4527–4538
Zhang X, Zheng Z, Shu B, Liu X, Zhang Z, Liu Y, Bai B, Hu Q, Mao P, Wang H (2013) Human astrocytic cells support persistent coxsackievirus B3 infection. J Virol 87(22):12407–12421
Shih SR, Weng KF, Stollar V, Li ML (2008) Viral protein synthesis is required for Enterovirus 71 to induce apoptosis in human glioblastoma cells. J Neurovirol 14(1):53–61
Beck MA, Chapman NM, McManus BM, Mullican JC, Tracy S (1990) Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am J Pathol 136(3):669–681
Wong WR, Chen YY, Yang SM, Chen YL, Horng JT (2005) Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71. Life Sci 78(1):82–90
Jiang L, Fantoni G, Couzens L, Gao J, Plant E, Ye Z, Eichelberger MC, Wan H (2016) Comparative efficacy of monoclonal antibodies that bind to different epitopes of the 2009 pandemic H1N1 influenza virus neuraminidase. J Virol 90(1):117–128
Wan H, Yang H, Shore DA, Garten RJ, Couzens L, Gao J, Jiang L, Carney PJ, Villanueva J, Stevens J et al (2015) Structural characterization of a protective epitope spanning A(H1N1)pdm09 influenza virus neuraminidase monomers. Nat Commun 6:6114
Zhu B, Li Y, Lin Z, Zhao M, Xu T, Wang C, Deng N (2016) Silver nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res Lett 11(1):198
Jheng JR, Lau KS, Tang WF, Wu MS, Horng JT (2010) Endoplasmic reticulum stress is induced and modulated by enterovirus 71. Cell Microbiol 12(6):796–813
Ong KC, Wong KT (2015) Understanding enterovirus 71 neuropathogenesis and its impact on other neurotropic enteroviruses. Brain Pathol 25(5):614–624
Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Tsao PN, Lee H, Lin MT, Lai HS, Chen CN, Lai DM et al (2005) GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer 113(6):920–927
Weinreb I, Goldstein D, Irish J, Perez-Ordonez B (2009) Expression patterns of Trk-A, Trk-B, GRP78, and p75NRT in olfactory neuroblastoma. Hum Pathol 40(9):1330–1335
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Mouse experiments were approved by the Ethics Committee of Guangzhou Medical University and performed according to the protocols and guidelines of the Experimental Animal Center of Guangzhou Medical University.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by the Technology Planning Project of Guangzhou (No. 201607010120), the Guangzhou Medical Health Science and Technology Project (No. 20161A010033), the Technology Planning Projects of Guangdong Province (No. 2014A020212697), and the China Postdoctoral Science Foundation (No. 2015M582366).
Authors’ contributions
ND and BZ designed the study. ND supervised the whole work and revised the manuscript. BZ, TX, ZL, YL, CW and MZ carried out the experiments. BZ and TX analyzed the data and drafted the manuscript. BZ and LH revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Zhu, B., Xu, T., Lin, Z. et al. Recombinant heat shock protein 78 enhances enterovirus 71 propagation in Vero cells and is induced in SK-N-SH cells during the infection. Arch Virol 162, 1649–1660 (2017). https://doi.org/10.1007/s00705-017-3287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3287-3