Abstract
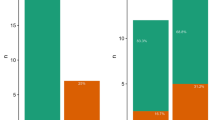
Beak and feather disease virus (BFDV) is a single-stranded DNA virus that is the etiological agent of beak and feather disease in both wild and captive parrots. Given that BFDV is globally recognized as a conservation threat for wild parrots, between 2011-2013, red-crowned parakeets (Cyanoramphus novaezelandiae, n = 229), which are endemic to New Zealand, were captured in mist nets on Tiritiri Matangi Island and Hauturu-o-Toi/Little Barrier Island (LBI), New Zealand, for disease surveillance. Blood and feathers from all birds were tested by PCR for BFDV, and full genomes were recovered and sequenced. A subset of blood samples (n = 96) were tested for antibodies to BFDV by the haemagglutination inhibition (HI) test. A further 238 feather samples were obtained from red-crowned parakeets from three sites in the Wellington region of the North Island, and these were screened for BFDV. The DNA-based prevalence of BFDV infection determined on Tiritiri Matangi Island was 1.09 % (CI 95 %, 0.1-3.9 %); on Hauturu-o-Toi/LBI, 4.4 % (95 % CI, 0.5 %-15.1 %); on Kapiti Island, 3.4 % (CI 95 %, 1.1-7.8 %); at the ZEALANDIA-Karori sanctuary, 1.6 % (95 % CI, 0-8.4 %); and on Matiu-Somes Island, 0 % (CI 95 %, 0-12.3 %). Seroprevalence for BFDV, indicating prior or current exposure, in the Tiritiri Matangi Island population, it was 2 % (CI 95 %, 0-10.1 %), and in the Hauturu-o-Toi/LBI population was 14 % (CI 95 %, 5.3-27.9 %). BFDV-positive birds showed no signs of clinical disease, with the exception of an individual bird obtained opportunistically from Shakespear Regional Park during the study period, which had classical signs of feather loss. Phylogenetic analysis of the 11 full genome sequences recovered from BFDV-positive red-crowned parakeets revealed evidence of ongoing viral flow between red-crowned parakeets and eastern rosellas (Platycercus eximius) in the Hauraki Gulf/Auckland region, with separate but closely related strains from the Wellington region of the North Island. This is the first study to report HI results for a New Zealand endemic parrot species, and the first epidemiological analysis of serial cross-sectional surveys in a BFDV-infected population of red-crowned parakeets in New Zealand. We postulate that although BFDV remains a threat to small, isolated or naïve populations of parrots globally, the low viral prevalence in this and other studies suggests that native parakeets in New Zealand may act as dead-end or spillover hosts.


Similar content being viewed by others
References
Collings DA, Collings BG, Julian L, Kurenbach B, Varsani A (2015) Genome sequences of beak and feather disease virus in urban rainbow lorikeets (Trichoglossus haematodus). Genome Announc 3(2):e00283-15
de Castro F, Bolker B (2005) Mechanisms of disease-induced extinction. Ecol Lett 8:117–126
Doneley RJ (2003) Acute beak and feather disease in juvenile African Grey parrots–an uncommon presentation of a common disease. Aust Vet J 81:206–207
Eastwood JR, Berg ML, Ribot RF, Raidal SR, Buchanan KL, Walder KR, Bennett AT (2014) Phylogenetic analysis of beak and feather disease virus across a host ring-species complex. PNAS 111:14153–14158
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Galbraith M, Cooper H (2013) Tiritiri Matangi—an overview of 25 years of ecological restoration. N Z J Ecol 37:258–260
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Ha HJ, Anderson IL, Alley MR, Springett BP, Gartrell BD (2007) The prevalence of beak and feather disease virus infection in wild populations of parrots and cockatoos in New Zealand. N Z Vet J 55:235–238
Ha HJ, Alley MR, Cahill JI, Howe L, Gartrell BD (2009) The prevalence of psittacine beak and feather disease virus infection in native parrots in New Zealand. N Z Vet J 57:50–52
Harkins GW, Martin DP, Christoffels A, Varsani A (2014) Towards inferring the global movement of beak and feather disease virus. Virology 450–451:24–33
Hess M, Scope A, Heincz U (2004) Comparitive sensitivity of polymerase chain reaction diagnosis of psittacine beak and feather disease on feather samples, cloacal swabs and blood from budgerigars (Melopsittacus undulates, Shaw 1805). Avian Pathol 33:477–481
Jackson B, Harvey C, Galbraith J, Robertson M, Warren K, Holyoake C, Julian L, Varsani A (2014) Clinical beak and feather disease virus infection in wild juvenile eastern rosellas of New Zealand; biosecurity implications for wildlife care facilities. N Z Vet J 62:297–301
Jackson B, Lorenzo A, Theuerkauf J, Barnaud A, Duval T, Guichard P, Bloc H, Baouma A, Stainton D, Kraberger S, Murphy S, Clark N, Dillon C, Knight T, Varsani A (2014) Preliminary surveillance for beak and feather disease virus in wild parrots of New Caledonia: implications of a reservoir species for Ouvea Parakeets. Emu 114:283–289
Julian L, Lorenzo A, Chenuet JP, Bonzon M, Marchal C, Vignon L, Collings DA, Walters M, Jackson B, Varsani A (2012) Evidence of multiple introductions of beak and feather disease virus into the Pacific islands of Nouvelle-Caledonie (New Caledonia). J Gen Virol 93:2466–2472
Julian L, Piasecki T, Chrzastek K, Walters M, Muhire B, Harkins GW, Martin DP, Varsani A (2013) Extensive recombination detected among beak and feather disease virus isolates from breeding facilities in Poland. J Gen Virol 94:1086–1095
Khalesi B, Bonne N, Stewart M, Sharp M, Raidal S (2005) A comparison of haemagglutination, haemagglutination inhibition and PCR for the detection of psittacine beak and feather disease virus infection and a comparison of isolates obtained from loriids. J Gen Virol 86:3039–3046
Kirkwood JK (1998) Population density and infectious disease at bird tables. Vet Rec 142:468
Kundu S, Faulkes CG, Greenwood AG, Jones CG, Kaiser P, Lyne OD, Black SA, Chowrimootoo A, Groombridge JJ (2012) Tracking viral evolution during a disease outbreak: the rapid and complete selective sweep of a circovirus in the endangered Echo parakeet. J Virol 86:5221–5229
Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462–2463
Massaro M, Ortiz-Catedral L, Julian L, Galbraith JA, Kurenbach B, Kearvell J, Kemp J, van Hal J, Elkington S, Taylor G, Greene T, van de Wetering J, van de Wetering M, Pryde M, Dilks P, Heber S, Steeves TE, Walters M, Shaw S, Potter J, Farrant M, Brunton DH, Hauber M, Jackson B, Bell P, Moorhouse R, McInnes K, Varsani A (2012) Molecular characterisation of beak and feather disease virus (BFDV) in New Zealand and its implications for managing an infectious disease. Arch Virol 157:1651–1663
Muhire BM, Varsani A, Martin DP (2014) SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PloS One 9:e108277
Ortiz-Catedral L, McInnes K, Hauber ME, Brunton DH (2009) First report of beak and feather disease virus (BFDV) in wild Red-fronted Parakeets (Cyanoramphus novaezelandiae) in New Zealand. Emu 109:244–247
Ortiz-Catedral L, Brunton DH (2010) Success of translocations of red-fronted parakeets Cyanoramphus novaezelandiae novaezelandiae from Little Barrier Island (Hauturu) to Motuihe Island, Auckland, New Zealand. Conserv Evid 7:21–26
Ortiz-Catedral L, Kurenbach B, Massaro M, McInnes K, Brunton DH, Hauber ME, Martin DP, Varsani A (2010) A new isolate of beak and feather disease virus from endemic wild red-fronted parakeets (Cyanoramphus novaezelandiae) in New Zealand. Arch Virol 155:613–620
Peters A, Patterson EI, Baker BGB, Holdsworth M, Sarker S, Ghorashi SA, Raidal SR (2014) Evidence of psittacine beak and feather disease virus spillover into wild critically endangered orange-bellied parrots (Neophema Chrysogaster). J Wildl Dis 50:288–296
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Raidal SR, McElnea CL, Cross GM (1993) Seroprevalence of psittacine beak and feather disease in wild psittacine birds in New South Wales. Aus Vet J 70:137–139
Raidal SR, Sabine M, Cross GM (1993) Laboratory diagnosis of psittacine beak and feather disease by haemagglutination and haemagglutination inhibition. Aust Vet J 70:133–137
Raidal SR (1995) Viral skin diseases of birds. Semin Avian Exotic Pet Med Viral Dis 4:72–82
Raidal SR, Cross GM (1995) Acute necrotizing hepatitis caused by experimental infection with psittacine beak and feather disease virus. J Avian Med Surg 9:36–40
Regnard GL, Boyes RS, Martin RO, Hitzeroth II, Rybicki EP (2015) Beak and feather disease viruses circulating in Cape parrots (Poicepahlus robustus) in South Africa. Arch Virol 160:47–54
Riddoch PA, Raidal SR, Cross GM (1996) Psittacine circovirus antibody detection and an update on the methods for diagnosis of psittacine beak and feather disease. Aust Vet Pract 26:134
Ritchie PA, Anderson IL, Lambert DM (2003) Evidence for specificity of psittacine beak and feather disease viruses among avian hosts. Virology 306:109–115
Robino P, Grego E, Rossi G, Bert E, Tramuta C, Stella M, Bertoni P, Nebbia P (2014) Molecular analysis and associated pathology of beak and feather diseae virus isolated in Italy from young Congo African grey parrots (Psittacus erithacus) with an “atypical peracute form” of the disease. Avian Pathol 43:333–344
Sarker S, Ghorashi SA, Forwood JK, Raidal SR (2013) Whole-genome sequences of two beak and feather disease viruses in the endangered swift parrot (Lathamus discolor). Genome Announc 1:e00842–e00913
Sarker S, Ghorashi SA, Forwood JK, Bent SJ, Peters A, Raidal SR (2014) Phylogeny of beak and feather disease virus in cockatoos demonstrates host generalism and multiple-variant infections within Psittaciformes. Virology 460:72–82
Sarker S, Patterson EI, Peters A, Baker GB, Forwood JK, Ghorashi SA, Holdsworth M, Baker R, Murray N, Raidal SR (2014) Mutability dynamics of an emergent single stranded DNA virus in a naive host. PloS One 9:e85370
Schoemaker NJ, Dorrestein GM, Latimer KS, Lumeij JT, Kik MJ, van der Hage MH, Campagnoli RP (2000) Severe leukopenia and liver necrosis in young African grey parrots (Psittacus erithacus erithacus) infected with psittacine circovirus. Avian Dis 44:470–478
Sergeant ESG (2015) Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease. http://epitools.ausvet.com.au
Todd D (2000) Circoviruses: immunosuppressive threats to avian species: a review. Avian Pathol 29:373–394
Varsani A, Regnard GL, Bragg R, Hitzeroth II, Rybicki EP (2011) Global genetic diversity and geographical and host-species distribution of beak and feather disease virus isolates. J Gen Virol 92:752–767
Acknowledgements
We wish to thank the following organisations for providing funds or in-kind support for this work: Auckland Zoo Conservation Fund, Murdoch University Research and Development Fund, Supporters of Tiritiri Matangi, Brian Mason Trust, Department of Conservation, and 360 Degrees Ferries. We also thank Ngati Manuhiri, Ngati Wai, Ngati Rehua, Ngati Paoa, SoTM and the Hauturu Supporters Trust for their support of work on the Hauraki Gulf Islands. Significant field assistance was generously provided through volunteers from the following organisations: Auckland Zoo, Massey University, SoTM, Department of Conservation and the Auckland Regional Council. Finally, we thank Bruce Harrison, Matt Maitland and Tim Lovegrove at Auckland Regional Council for their ongoing observations and providing the BFDV-positive specimen from Shakespear Regional Park.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jackson, B., Varsani, A., Holyoake, C. et al. Emerging infectious disease or evidence of endemicity? A multi-season study of beak and feather disease virus in wild red-crowned parakeets (Cyanoramphus novaezelandiae) . Arch Virol 160, 2283–2292 (2015). https://doi.org/10.1007/s00705-015-2510-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2510-3