Abstract
Background
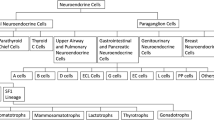
The WHO 2021 introduced the term pituitary neuroendocrine tumours (PitNETs) for pituitary adenomas and incorporated transcription factors for subtyping, prompting the need for fresh diagnostic methods. Current biomarkers struggle to distinguish between high- and low-risk non-functioning PitNETs. We explored if radiomics can enhance preoperative decision-making.
Methods
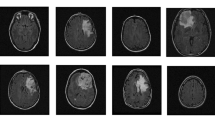
Pre-treatment magnetic resonance (MR) images of patients who underwent surgery between 2015 and 2019 with available WHO 2021 classification were used. The tumours were manually segmented on the T1w, T1-contrast enhanced, and T2w images using 3D Slicer. One hundred Pyradiomic features were extracted from each MR sequence. Models were built to classify (1) somatotroph and gonadotroph PitNETs and (2) high- and low-risk subtypes of non-functioning PitNETs. Feature were selected independently from the MR sequences and multi-sequence (combining data from more than one MR sequence) using Boruta and Pearson correlation. Support vector machine (SVM), logistic regression (LR), random forest (RF), and multi-layer perceptron (MLP) were the classifiers used. Data imbalance was addressed using the Synthetic Minority Oversampling TEchnique (SMOTE). Performance of the models were evaluated using area under the receiver operating curve (AUC), accuracy, sensitivity, and specificity.
Results
A total of 222 PitNET patients (train, n = 149; test, n = 73) were enrolled in this retrospective study. Multi-sequence-based LR model discriminated best between somatotroph and gonadotroph PitNETs, with a test AUC of 0.84, accuracy of 0.74, specificity of 0.81, and sensitivity of 0.70. Multi-sequence-based MLP model perfomed best for the high- and low-risk non-functioning PitNETs, achieving a test AUC of 0.76, accuracy of 0.67, specificity of 0.72, and sensitivity of 0.66.
Conclusions
Utilizing pre-treatment MRI and radiomics holds promise for distinguishing high-risk from low-risk non-functioning PitNETs based on the latest WHO classification. This could assist neurosurgeons in making critical decisions regarding surgery or alternative management strategies for PitNETs after further clinical validation.




Similar content being viewed by others
Data availability
Available on reasonable request.
Code availability
Codes will be made available on GitHub shortly.
Abbreviations
- WHO:
-
World Health Organization
- MR:
-
Magnetic resonance
- PitNETs:
-
Pituitary neuroendocrine tumours
- SVM:
-
Support vector machine
- LR:
-
Logistic regression
- RF:
-
Random forest
- MLP:
-
Multi-layer perceptron
- SMOTE:
-
Synthetic Minority Oversampling TEchnique
- NCA:
-
Null cell adenoma
- GA:
-
Gonadotroph adenomas
- SCA:
-
Silent corticotroph adenoma
- SPA:
-
Silent PIT-1 positive adenomas
- T1w:
-
T1-weighted MRI
- T2w:
-
T2-weighted MRI
- T1-CE:
-
T1-gadolinium contrast enhanced MRI
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray level run length matrix
- GLSZM:
-
Gray level size zone matrix
- GLDM:
-
Gray level dependence matrix
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the receiver operating characteristic (ROC) curve
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- ASL:
-
Arterial spin labelling
- PIT1:
-
Pituitary-specific positive transcription factor 1
- TPIT:
-
T-box transcription factor
- SF1:
-
Steroidogenic factor
- ER:
-
Estrogen receptor
- SA:
-
Somatotroph adenoma
- GATA3:
-
GATA binding protein 3
References
Ahmadi J, North CM, Segall HD, Zee CS, Weiss MH (1986) Cavernous sinus invasion by pituitary adenomas. AJR Am J Roentgenol 146(2):257–262
Almeida JP, Stephens CC, Eschbacher JM et al (2019) Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: a case series from two pituitary centers. Pituitary 22(5):514–519
Asa SL, Mete O, Perry A, Osamura RY (2022) Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol 33(1):6–26
Asa SL, Mete O, Riddle ND, Perry A (2023) Multilineage pituitary neuroendocrine tumors (PitNETs) Expressing PIT1 and SF1. Endocr Pathol 34(3):273–278
Asha MJ, Takami H, Velasquez C, Oswari S, Almeida JP, Zadeh G, Gentili F (2019) Long-term outcomes of transsphenoidal surgery for management of growth hormone-secreting adenomas: single-center results. J Neurosurg 11:1–11. https://doi.org/10.3171/2019.6.JNS191187
Cazabat L, Dupuy M, Boulin A, Bernier M, Baussart B, Foubert L, Raffin-Sanson M-L, Caron P, Bertherat J, Gaillard S (2014) Silent, but not unseen: multimicrocystic aspect on T2-weighted MRI in silent corticotroph adenomas. Clin Endocrinol (Oxf) 81(4):566–572
Cordeiro D, Xu Z, Mehta GU et al (2018) Hypopituitarism after gamma knife radiosurgery for pituitary adenomas: a multicenter, international study. J Neurosurg 131(4):1188–1196
Fan Y, Jiang S, Hua M, Feng S, Feng M, Wang R (2019) Machine learning-based radiomics predicts radiotherapeutic response in patients with acromegaly. Front Endocrinol 10:588
Goyal-Honavar A, Sarkar S, Asha HS, Kapoor N, Thomas R, Balakrishnan R, Chacko G, Chacko AG (2021) Impact of experience on outcomes after endoscopic transsphenoidal surgery for acromegaly. World Neurosurgery 151:e1007–e1015
Goyal-Honavar A, Sarkar S, Hesarghatta A, Kapoor N, Balakrishnan R, Vanjare H, Chacko G, Chacko A (2021) A clinicoradiological analysis of silent corticotroph adenomas after the introduction of pituitary-specific transcription factors. Acta Neurochir. https://doi.org/10.1007/s00701-021-04911-2
Haddad AF, Young JS, Oh T et al (2020) Clinical characteristics and outcomes of null-cell versus silent gonadotroph adenomas in a series of 1166 pituitary adenomas from a single institution. Neurosurg Focus 48(6):E13
Jahangiri A, Wagner JR, Pekmezci M, Hiniker A, Chang EF, Kunwar S, Blevins L, Aghi MK (2013) A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery 73(1):8–18
J Jia L Meng G Song S Sun C Li J Tian Y Zhang 2020 Prediction of response to stereotactic radiotherapy for nonfunctioning pituitary adenoma using radiomic feature https://doi.org/10.21203/rs.2.21209/v1
Kiseljak-Vassiliades K, Carlson NE, Borges MT, Kleinschmidt-DeMasters BK, Lillehei KO, Kerr JM, Wierman ME (2015) Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 49(1):231–241
Langlois F, Lim DST, Yedinak CG, Cetas I, McCartney S, Cetas J, Dogan A, Fleseriu M (2018) Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary 21(1):32–40
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251
MacFarlane J, Gillett D, Koulouri O, Bashari W, Casey R, Gurnell M (2022) Radiomics as a tool for risk stratification of non-functioning pituitary adenomas following primary surgery. Endocr Abstr. https://doi.org/10.1530/endoabs.86.OC3.5
Machado LF, Elias PCL, Moreira AC, Dos Santos AC, Murta Junior LO (2020) MRI radiomics for the prediction of recurrence in patients with clinically non-functioning pituitary macroadenomas. Comput Biol Med 124:103966
Mendi BAR, Batur H, Çay N, Çakır BT (2023) Radiomic analysis of preoperative magnetic resonance imaging for the prediction of pituitary adenoma consistency. Acta Radiol 64(8):2470–2478
Mete O, Lopes MB (2017) Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr Pathol 28(3):228–243
Micko ASG, Wöhrer A, Wolfsberger S, Knosp E (2015) Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. JNS 122(4):803–811
Osborn AG, Louis DN, Poussaint TY, Linscott LL, Salzman KL (2022) The 2021 World Health Organization Classification of Tumors of the Central Nervous System: what neuroradiologists need to know. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A7462
Peng A, Dai H, Duan H, Chen Y, Huang J, Zhou L, Chen L (2020) A machine learning model to precisely immunohistochemically classify pituitary adenoma subtypes with radiomics based on preoperative magnetic resonance imaging. Eur J Radiol 125:108892
Rui W, Qiao N, Wu Y, Zhang Y, Aili A, Zhang Z, Ye H, Wang Y, Zhao Y, Yao Z (2022) Radiomics analysis allows for precise prediction of silent corticotroph adenoma among non-functioning pituitary adenomas. Eur Radiol 32(3):1570–1578
Wang H, Chang J, Zhang W et al (2023) Radiomics model and clinical scale for the preoperative diagnosis of silent corticotroph adenomas. J Endocrinol Invest 46(9):1843–1854
Won SY, Lee N, Park YW, Ahn SS, Ku CR, Kim EH, Lee S-K (2022) Quality reporting of radiomics analysis in pituitary adenomas: promoting clinical translation. Br J Radiol 95(1139):20220401
Zhang Y, Luo Y, Kong X, Wan T, Long Y, Ma J (2022) A preoperative MRI-based radiomics-clinicopathological classifier to predict the recurrence of pituitary macroadenoma within 5 years. Front Neurol 12:780628
Zhang S, Song G, Zang Y, Jia J, Wang C, Li C, Tian J, Dong D, Zhang Y (2018) Non-invasive radiomics approach potentially predicts non-functioning pituitary adenomas subtypes before surgery. Eur Radiol 28(9):3692–3701. https://doi.org/10.1007/s00330-017-5180-6
Acknowledgements
The authors acknowledge the support provided by Mr. Reji towards data anonymization and transfer of images.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: AGC, AJ, GC, DD, BKS, SPP, HMTT; data collection: SA, AGH, JAS. Author; analysis, and interpretation of results: SA, AGH, AGC, AJ, GC, DD, JAS, BKS, SPP, HMTT; draft manuscript preparation: SA, AGH; revised manuscript critically for important intellectual content: AGC, AJ, GC, DD, BKS, SPP, HMTT. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted in accordance with the 1964 Declaration of Helsinki and was approved by the Institutional Review Board with the need for informed consent waived due to the retrospective nature of the study.
Consent to participate
Waiver granted due to retrospective nature of study.
Consent for publication
All authors have consented to publishing this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sathya A and Abhijit Goyal-Honavar are equal contributing first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathya A, Goyal-Honavar, A., Chacko, A.G. et al. Is radiomics a useful addition to magnetic resonance imaging in the preoperative classification of PitNETs?. Acta Neurochir 166, 91 (2024). https://doi.org/10.1007/s00701-024-05977-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-05977-4