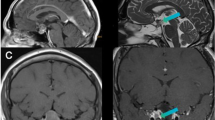
Abstract
Background
It is currently unclear if there are subsets of patients undergoing transsphenoidal surgery (TSS) in which intraoperative high-field magnetic resonance imaging (3T-iMRI) is particularly advantageous. We aimed to investigate whether a radiological grading scale predicts the utility of 3T-iMRI in pituitary adenoma (PA) TSS.
Methods
From a prospective registry, patients who underwent endoscopic TSS for PA using 3T-iMRI were identified. Adenomas were graded using the Zurich Pituitary Score (ZPS). We assessed improvement after 3T-iMRI in terms of gross total resection (GTR), residual volume (RV), and extent of resection (EOR).
Results
Among 95 patients, rates of conversion to GTR after 3T-iMRI decreased steadily from 33% for grade I to 0% for grade IV adenomas, with a statistically significant conversion rate only for grade I (p = 0.008) and grade II (p < 0.001). All grade I adenomas were completely resected after 3T-iMRI. Median RV change was statistically significant for grades I to III, but not for grade IV (p = 0.625). EOR improvement ranged from a median change of 0.0% (IQR 0.0–4.5%) for grade I to 4.4% (IQR 0.0–9.0%) for grade IV, with a significant improvement only for grades I to III (p < 0.05).
Conclusions
Interestingly, this study shows that clinical utility of 3T-iMRI is highest in the more “simple” adenomas (ZPS grades I–II) than for the more “complex” ones (ZPS grade III–IV). Grade I adenomas are amenable to GTR if 3T-iMRI is implemented. In grade III adenomas, EOR and RV can be improved to clinically relevant levels. Conversely, in grade IV adenomas, 3T-iMRI may be of limited use.




Similar content being viewed by others
References
Barker FG, Klibanski A, Swearingen B (2003) Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab 88(10):4709–4719
Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, Wang C, Swerdloff RS, Kelly DF (2008) Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery 63(4):709–719
Hardy J, Vezina JL (1976) Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 15:261–273
Mehta GU, Oldfield EH (2012) Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg 116(6):1299–1303
Przybylowski CJ, Dallapiazza RF, Williams BJ, Pomeraniec IJ, Xu Z, Payne SC, Laws ER, Jane JA (2016) Primary versus revision transsphenoidal resection for nonfunctioning pituitary macroadenomas: matched cohort study. J Neurosurg 126(3):889–896
Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G (2018) Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg 1–8. https://doi.org/10.3171/2017.12.JNS172318
Chen C-J, Ironside N, Pomeraniec IJ, Chivukula S, Buell TJ, Ding D, Taylor DG, Dallapiazza RF, Lee C-C, Bergsneider M (2017) Microsurgical versus endoscopic transsphenoidal resection for acromegaly: a systematic review of outcomes and complications. Acta Neurochir 159(11):2193–2207
Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH (2016) Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg 96:36–46
Micko ASG, Wöhrer A, Wolfsberger S, Knosp E (2015) Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg 122(4):803–811
Elhadi AM, Hardesty DA, Zaidi HA, Kalani MYS, Nakaji P, White WL, Preul MC, Little AS (2015) Evaluation of surgical freedom for microscopic and endoscopic transsphenoidal approaches to the sella. Neurosurgery 11(Suppl 2):69–78 discussion 78-79
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617 discussion 617-618
Serra C, Maldaner N, Muscas G, Staartjes V, Pangalu A, Holzmann D, Soyka M, Schmid C, Regli L (2017) The changing sella: internal carotid artery shift during transsphenoidal pituitary surgery. Pituitary:1–7
Serra C, Staartjes VE, Maldaner N, Muscas G, Akeret K, Holzmann D, Soyka MB, Schmid C, Regli L (2018) Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir. https://doi.org/10.1007/s00701-018-3690-x
Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH (2012) Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 15(3):450–463
Meij BP, Lopes M-BS, Ellegala DB, Alden TD, Laws ER (2002) The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg 96(2):195–208
Mooney MA, Hardesty DA, Sheehy JP, Bird R, Chapple K, White WL, Little AS (2016) Interrater and intrarater reliability of the Knosp scale for pituitary adenoma grading. J Neurosurg 126(5):1714–1719
Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, Vogelbaum MA (2014) Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg 121(5):1115–1123
Jahangiri A, Wagner J, Han SW et al (2014) Morbidity of repeat transsphenoidal surgery assessed in more than 1000 operations. J Neurosurg 121(1):67–74
Negm HM, Al-Mahfoudh R, Pai M, Singh H, Cohen S, Dhandapani S, Anand VK, Schwartz TH (2016) Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J Neurosurg:1–12
Sughrue ME, Chang EF, Gabriel RA, Aghi MK, Blevins LS (2011) Excess mortality for patients with residual disease following resection of pituitary adenomas. Pituitary 14(3):276–283
Nelson AT, Tucker HSG, Becker DP (1984) Residual anterior pituitary function following transsphenoidal resection of pituitary macroadenomas. J Neurosurg 61(3):577–580
Soyka MB, Serra C, Regli L, Meier E, Holzmann D (2017) Long-term olfactory outcome after nasoseptal flap reconstructions in midline skull base surgery. Am J Rhinol Allergy 31(5):334–337
Staartjes VE, Stricker S, Muscas G, Maldaner N, Holzmann D, Burkhardt J-K, Seifert B, Schmid C, Serra C, Regli L (2018) Intraoperative unfolding and postoperative pruning of the pituitary gland after transsphenoidal surgery for pituitary adenoma: a volumetric and endocrinological evaluation. Endocrine. https://doi.org/10.1007/s12020-018-1758-2
Webb SM, Rigla M, Wägner A, Oliver B, Bartumeus F (1999) Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab 84(10):3696–3700
Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M (2014) Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir 156(12):2233–2243
Buchfelder M, Schlaffer S-M (2012) Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons. Endocrine 42(3):483–495
Chittiboina P (2017) iMRI during transsphenoidal surgery (TSS). Neurosurg Clin N Am 28(4):499–512
Coburger J, König R, Seitz K, Bäzner U, Wirtz CR, Hlavac M (2014) Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 120(2):346–356
Fomekong E, Duprez T, Docquier M-A, Ntsambi G, Maiter D, Raftopoulos C (2014) Intraoperative 3T MRI for pituitary macroadenoma resection: initial experience in 73 consecutive patients. Clin Neurol Neurosurg 126:143–149
Netuka D, Masopust V, Belšán T, Kramář F, Beneš V (2011) One year experience with 3.0 T intraoperative MRI in pituitary surgery. Acta Neurochir Suppl 109:157–159
Nimsky C, von Keller B, Ganslandt O, Fahlbusch R (2006) Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery 59(1):105–114
Paľa A, Knoll A, Brand C, Etzrodt-Walter G, Coburger J, Wirtz CR, Hlaváč M (2017) The value of intraoperative MRI in endoscopic and microsurgical transsphenoidal pituitary adenoma resection. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.02.132
Pamir MN (2011) 3 T ioMRI: the Istanbul experience. Acta Neurochir Suppl 109:131–137
Serra C, Burkhardt J-K, Esposito G, Bozinov O, Pangalu A, Valavanis A, Holzmann D, Schmid C, Regli L (2016) Pituitary surgery and volumetric assessment of extent of resection: a paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg Focus 40(3):E17
Sylvester PT, Evans JA, Zipfel GJ et al (2015) Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 18(1):72–85
Szerlip NJ, Zhang Y-C, Placantonakis DG, Goldman M, Colevas KB, Rubin DG, Kobylarz EJ, Karimi S, Girotra M, Tabar V (2011) Transsphenoidal resection of sellar tumors using high-field intraoperative magnetic resonance imaging. Skull Base 21(4):223–232
Zaidi HA, De Los RK, Barkhoudarian G, Litvack ZN, Bi WL, Rincon-Torroella J, Mukundan S, Dunn IF, Laws ER (2016) The utility of high-resolution intraoperative MRI in endoscopic transsphenoidal surgery for pituitary macroadenomas: early experience in the advanced multimodality image guided operating suite. Neurosurg Focus 40(3):E18
Schwartz TH, Stieg PE, Anand VK (2006) Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Oper Neurosurg 58(suppl_1):ONS-44-ONS-51
Kuo JS, Barkhoudarian G, Farrell CJ, Bodach ME, Tumialan LM, Oyesiku NM, Litvack Z, Zada G, Patil CG, Aghi MK (2016) Congress of Neurological Surgeons systematic review and evidence-based guideline on surgical techniques and technologies for the management of patients with nonfunctioning pituitary adenomas. Neurosurgery 79(4):E536–E538
Stienen MN, Fierstra J, Pangalu A, Regli L, Bozinov O (2018) The Zurich checklist for safety in the intraoperative magnetic resonance imaging suite: technical note. Oper Neurosurg Hagerstown Md. https://doi.org/10.1093/ons/opy205
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624):806–808
Streitberg B, Röhmel J (1986) Exact distributions for permutation and rank tests: an introduction to some recently published algorithms. Stat Softw Newsl 12(1):10–17
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Colao A, Attanasio R, Pivonello R, Cappabianca P, Cavallo LM, Lasio G, Lodrini A, Lombardi G, Cozzi R (2006) Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab 91(1):85–92
O’Sullivan EP, Woods C, Glynn N, Behan LA, Crowley R, O’Kelly P, Smith D, Thompson CJ, Agha A (2009) The natural history of surgically treated but radiotherapy-naïve nonfunctioning pituitary adenomas. Clin Endocrinol 71(5):709–714
Schwyzer L, Starke RM, Jane JA, Oldfield EH (2014) Percent reduction of growth hormone levels correlates closely with percent resected tumor volume in acromegaly. J Neurosurg 122(4):798–802
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Cantonal Ethics Committee Zürich, KEK St-V-Nr 2015-0142) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pituitaries
Electronic supplementary material
701_2019_4018_MOESM1_ESM.r
Supplementary Methods 1 Supplementary Content 1. R Code for the statistical analysis and figure rendering. The code was executed in R Version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria) on a machine running Windows 10 (Microsoft Corp., Redmond, WA, USA). (R 17 kb)

Supplementary Figure 1
Extent of resection (EOR) in percent as measured (A) on intraoperative magnetic resonance imaging (MRI) and (B) on the 3-month follow-up MRI, stratified by Zurich Pituitary Score. An extreme outlier (Grade II) presenting with an intraoperative EOR of 19.6% is not depicted in order to preserve scale. (JPG 8296 kb)
Rights and permissions
About this article
Cite this article
Staartjes, V.E., Serra, C., Maldaner, N. et al. The Zurich Pituitary Score predicts utility of intraoperative high-field magnetic resonance imaging in transsphenoidal pituitary adenoma surgery. Acta Neurochir 161, 2107–2115 (2019). https://doi.org/10.1007/s00701-019-04018-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04018-9