Abstract
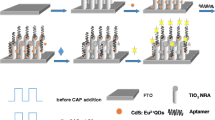
A method is described for photoelectrochemical determination of chloramphenicol (CLOA). It is based on the use of (a) aptamers protected with photoactive WS2 nanosheets, and (b) DNase I-assisted target recycling. The DNA aptamer without label was employed for recognition of CLOA. In the absence of CLOA, the aptamer is adsorbed on the surface of WS2. This leads to a decrease of photocurrent due to the steric-hindrance effect of aptamer DNA. The adsorption of WS2 also protects the aptamer from digestion by DNase. In the presence of CLOA, the aptamer will be desorbed from the WS2 surface due to formation of an aptamer/CLOA conjugate. This results in an increased photocurrent due to a decreased amount of aptamer DNA on the electrode surface. The increase of photocurrent can be further improved by applying DNase triggered catalytic recycling of CLOA. Under optimal experimental conditions, the response is linear 10 pM – 10 nM CLOA concentration range, with a 3.6 pM lower detection limit (at 3σ). This method is acceptably selective, accurate and stable. It was applied to the determination of CLOA in spiked milk samples and gave satisfactory results.

A simple and sensitive photoelectrochemical apta-biosensor was fabricated for chloramphenicol detection. In this work, WS2 nanosheets were employed as photoactive material, and DNase I catalytic chloramphenicol recycling strategy was adopted to amplify the detection signal.




Similar content being viewed by others
References
Wang L, Zhang Y, Gao X, Duan Z, Wang S (2010) Determination of chloramphenicol residues in milk by enzyme-liinked immunosorbent assay: improvement by biotin-streptavidin-amplified system. J Agric Food Chem 58(6):3265–3270. https://doi.org/10.1021/jf903940h
Miao Y-B, Ren H-X, Gan N, Cao Y, Li T, Chen Y (2016) Fluorescent aptasensor for chloramphenicol detection using DIL-encapsulated liposome as nanotracer. Biosens Bioelectron 81:454–459. https://doi.org/10.1016/j.bios.2016.03.034
Jianbing W, Linyao C, Peipei M, Yanbin L, Huizhong W (2012) Determination of chloramphenicol in aquatic products by graphene-based SPE coupled with HPLC-MS/MS. J Sep Sci 35(24):3586–3592. https://doi.org/10.1002/jssc.201200617
Xiao L, Xu R, Yuan Q, Wang F (2017) Highly sensitive electrochemical sensor for chloramphenicol based on MOF derived exfoliated porous carbon. Talanta 167:39–43. https://doi.org/10.1016/j.talanta.2017.01.078
Feng X, Gan N, Zhang H, Yan Q, Li T, Cao Y, Hu F, Yu H, Jiang Q (2015) A novel “dual-potential” electrochemiluminescence aptasensor array using CdS quantum dots and luminol-gold nanoparticles as labels for simultaneous detection of malachite green and chloramphenicol. Biosens Bioelectron 74:587–593. https://doi.org/10.1016/j.bios.2015.06.048
Liu Y, Yan K, Okoth OK, Zhang J (2015) A label-free photoelectrochemical aptasensor based on nitrogen-doped graphene quantum dots for chloramphenicol determination. Biosens Bioelectron 74:1016–1021. https://doi.org/10.1016/j.bios.2015.07.067
Zhao W-W, Xu J-J, Chen H-Y (2015) Photoelectrochemical bioanalysis: the state of the art. Chem Soc Rev 44(3):729–741. https://doi.org/10.1039/c4cs00228h
Hun X, Wang S, Wang S, Zhao J, Luo X (2017) A photoelectrochemical sensor for ultrasensitive dopamine detection based on single-layer NanoMoS2 modified gold electrode. Sensors Actuators B Chem 249:83–89. https://doi.org/10.1016/j.snb.2017.04.065
Qin C, Bai X, Zhang Y, Gao K (2018) Photoelectrochemical CdSe/TiO2 nanotube array microsensor for high-resolution in-situ detection of dopamine. Microchim Acta 185(5):278. https://doi.org/10.1007/s00604-018-2788-4
Wang Y, Wang P, Wu Y, Di J (2018) A cathodic “signal-on” photoelectrochemical sensor for Hg2+ detection based on ion-exchange with ZnS quantum dots. Sensors Actuators B Chem 254:910–915. https://doi.org/10.1016/j.snb.2017.07.149
Shi Y, Zhang G, Li J, Zhang Y, Yu Y, Wei Q (2017) Photoelectrochemical determination of Hg(II) via dual signal amplification involving SPR enhancement and a folding-based DNA probe. Microchim Acta 184(5):1379–1387. https://doi.org/10.1007/s00604-017-2141-3
Yang Z, Shi Y, Liao W, Yin H, Ai S (2016) A novel signal-on photoelectrochemical biosensor for detection of 5-hydroxymethylcytosine based on in situ electron donor producing strategy and all wavelengths of light irradiation. Sensors Actuators B Chem 223:621–625. https://doi.org/10.1016/j.snb.2015.09.159
Wang M, Yin H, Zhou Y, Han J, He T, Cui L, Ai S (2018) Photoelectrochemical biosensor for microRNA detection based on multiple amplification strategies. Microchim Acta 185(5):257. https://doi.org/10.1007/s00604-018-2808-4
Zhou Q, Lin Y, Shu J, Zhang K, Yu Z, Tang D (2017) Reduced graphene oxide-functionalized FeOOH for signal-on photoelectrochemical sensing of prostate-specific antigen with bioresponsive controlled release system. Biosens Bioelectron 98:15–21. https://doi.org/10.1016/j.bios.2017.06.033
Cheng W, Pan J, Yang J, Zheng Z, Lu F, Chen Y, Gao W (2018) A photoelectrochemical aptasensor for thrombin based on the use of carbon quantum dot-sensitized TiO2 and visible-light photoelectrochemical activity. Microchim Acta 185(5):263. https://doi.org/10.1007/s00604-018-2800-z
Liu X-P, Xie X-L, Wei Y-P, Mao C-j, Chen J-S, Niu H-L, Song J-M, Jin B-K (2018) Photoelectrochemical immunoassay for human interleukin 6 based on the use of perovskite-type LaFeO3 nanoparticles on fluorine-doped tin oxide glass. Microchim Acta 185(1):52. https://doi.org/10.1007/s00604-017-2554-z
Zhang X, Liu M, Liu H, Zhang S (2014) Low-toxic Ag2S quantum dots for photoelectrochemical detection glucose and cancer cells. Biosens Bioelectron 56:307–312. https://doi.org/10.1016/j.bios.2014.01.033
Tan C, Zhang H (2015) Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem Soc Rev 44(9):2713–2731. https://doi.org/10.1039/c4cs00182f
Tan Y, Li M, Ye X, Wang Z, Wang Y, Li C (2018) Ionic liquid auxiliary exfoliation of WS2 nanosheets and the enhanced effect of hollow gold nanospheres on their photoelectrochemical sensing towards human epididymis protein 4. Sensors Actuators B Chem 262:982–990. https://doi.org/10.1016/j.snb.2018.02.066
Xi Q, Zhou D-M, Kan Y-Y, Ge J, Wu Z-K, Yu R-Q, Jiang J-H (2014) Highly sensitive and selective strategy for microRNA detection based on WS2 nanosheet mediated fluorescence quenching and duplex-specific nuclease signal amplification. Anal Chem 86(3):1361–1365. https://doi.org/10.1021/ac403944c
Zuo X, Zhang H, Zhu Q, Wang W, Feng J, Chen X (2016) A dual-color fluorescent biosensing platform based on WS2 nanosheet for detection of Hg2+ and ag+. Biosens Bioelectron 85:464–470. https://doi.org/10.1016/j.bios.2016.05.044
Zhu C, Zeng Z, Li H, Li F, Fan C, Zhang H (2013) Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. JACS 135(16):5998–6001. https://doi.org/10.1021/ja4019572
Dechtrirat D, Yingyuad P, Prajongtat P, Chuenchom L, Sriprachuabwong C, Tuantranont A, Tang I-M (2018) A screen-printed carbon electrode modified with gold nanoparticles, poly(3,4-ethylenedioxythiophene), poly(styrene sulfonate) and a molecular imprint for voltammetric determination of nitrofurantoin. Microchim Acta 185(5):261. https://doi.org/10.1007/s00604-018-2797-3
Liu S, Lai G, Zhang H, Yu A (2017) Amperometric aptasensing of chloramphenicol at a glassy carbon electrode modified with a nanocomposite consisting of graphene and silver nanoparticles. Microchim Acta 184(5):1445–1451. https://doi.org/10.1007/s00604-017-2138-y
Pesci FM, Sokolikova MS, Grotta C, Sherrell PC, Reale F, Sharda K, Ni N, Palczynski P, Mattevi C (2017) MoS2/WS2 heterojunction for photoelectrochemical water oxidation. ACS Catal 7(8):4990–4998. https://doi.org/10.1021/acscatal.7b01517
Teo WZ, Chng ELK, Sofer Z, Pumera M (2014) Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem Eur J 20(31):9627–9632. https://doi.org/10.1002/chem.201402680
Tan Z, Xu H, Li G, Yang X, Choi MMF (2015) Fluorescence quenching for chloramphenicol detection in milk based on protein-stabilized Au nanoclusters. Spectrochim Acta A 149:615–620. https://doi.org/10.1016/j.saa.2015.04.109
Chang H, Lv J, Zhang H, Zhang B, Wei W, Qiao Y (2017) Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens Bioelectron 87:579–586. https://doi.org/10.1016/j.bios.2016.09.002
Karaseva NA, Ermolaeva TN (2012) A piezoelectric immunosensor for chloramphenicol detection in food. Talanta 93:44–48. https://doi.org/10.1016/j.talanta.2011.12.047
Bai X, Qin C, Huang X (2016) Voltammetric determination of chloramphenicol using a carbon fiber microelectrode modified with Fe3O4 nanoparticles. Microchim Acta 183(11):2973–2981. https://doi.org/10.1007/s00604-016-1945-x
Wang A, Zhang L, Fang Y (1999) Determination and separation of chloramphenicol and its hydrolysate in eye-drops by capillary zone electrophoresis with amperometric detection. Anal Chim Acta 394(2):309–316. https://doi.org/10.1016/S0003-2670(99)00314-1
Pilehvar S, Mehta J, Dardenne F, Robbens J, Blust R, De Wael K (2012) Aptasensing of chloramphenicol in the presence of its analogues: reaching the maximum residue limit. Anal Chem 84(15):6753–6758. https://doi.org/10.1021/ac3012522
Feng YX, Bing LN, Qun LH (2012) Post-chemiluminescence determination of chloramphenicol based on luminol-potassium periodate system. Luminescence 27(3):217–222. https://doi.org/10.1002/bio.1335
Yan L, Luo C, Cheng W, Mao W, Zhang D, Ding S (2012) A simple and sensitive electrochemical aptasensor for determination of chloramphenicol in honey based on target-induced strand release. J Electroanal Chem 687:89–94. https://doi.org/10.1016/j.jelechem.2012.10.016
Wang Y, Bian F, Qin X, Wang Q (2018) Visible light photoelectrochemical aptasensor for chloramphenicol by using a TiO2 nanorod array sensitized with Eu(III)-doped CdS quantum dots. Microchim Acta 185(3):161. https://doi.org/10.1007/s00604-018-2711-z
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.21775090), the Natural Science Foundation of Shandong province, China (No. ZR2016BM10, ZR2018MB028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 193 kb)
Rights and permissions
About this article
Cite this article
Zhou, Y., Sui, C., Yin, H. et al. Tungsten disulfide (WS2) nanosheet-based photoelectrochemical aptasensing of chloramphenicol. Microchim Acta 185, 453 (2018). https://doi.org/10.1007/s00604-018-2970-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2970-8