Abstract
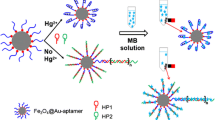
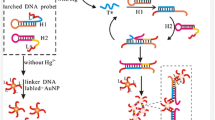
A method was developed for the determination of mercuric ion Hg(II). It is based on hybridization chain reaction (HCR) and surface-enhanced Raman scattering (SERS). Raman signal DNA and streptavidin were self-assembled on gold nanoparticles as a novel signal nanoprobe (AuNP-sDNA). A thymine-mercury(II)-thymine structure was immobilized on magnetic beads (MBs). The HCR makes use of two hairpin probes that are initiated by the trigger DNA to form a stable nicked dsDNA structure (MB-TS-hDNAs). A large number of the binding sites is provided to connect the signal nanoprobe. The stable sandwich structure (MB-TS-hDNA/AuNP-sDNA) was isolated by applying a magnetic field and used in the amplification step. In this way, Hg(II) can be determined sensitively after multiple signal amplification. The SERS signal, measured at 1499 cm−1, increases linearly in the 0.1 pM to 10 nM Hg(II) concentration range, and the limit of detection is 0.08 pM (at an S/N ratio of 3). The method was applied to the detection of Hg(II) in spiked environment water samples, with recoveries ranging from 96 to 119%.

Schematic of a method based on the use of a stable T-Hg(II)-T structure and a self-assembled nanoprobe. It was applied to the trace Hg(II) detection based on hybridization chain reaction (HCR) and surface-enhanced Raman scattering (SERS).





Similar content being viewed by others
References
Henriques JF, Tavares PC, Correia-Dos-Santos MM, Trancoso MA, Santos-Reis M, Branquinho C (2014) Monitoring Hg and Cd contamination using red swamp crayfish (procambarus clarkii): implications for wetland food chain contamination. Water Air Soil Pollut 225:2210
Jarup L (2003) Treatment of heavy metal contamination. Br Med Bull 68:167–182
Bordajandi LR, Gómez G, Abad E, Rivera J, Del MFM, Blasco J, Gonzalez MJ (2004) Survey of persistent organochlorine contaminants (PCBs, PCDD/Fs, and PAHs), heavy metals (Cu, Cd, Zn, Pb, and Hg), and arsenic in food samples from Huelva (Spain): levels and health implications. J Agric Food Chem 52:992–1001
Gandhi N, Tang RWK, Bhavsar SP, Arhonditsis GB (2014) Fish mercury levels appear to be increasing lately: a report from 40 years of monitoring in the province of Ontario, Canada. Environ Sci Technol 48:5404–5414
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203
Ariza ME, Williams MV (1999) Lead and mercury mutagenesis: type of mutation dependent upon metal concentration. J Biochem Mol Toxicol 13:107–112
Cantoni O, Christie NT, Swann A, Drath DB, Costa M (1984) Mechanism of HgCl2 cytotoxicity in cultured mammalian cells. Mol Pharm 26:360–368
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Onyido I, Norris AR, Buncel E (2004) Biomolecule—mercury interactions: modalities of DNA base—mercury binding mechanisms. remediation strategies. Cheminform 104:5911–5929
Schneider L, Peleja RP, Kluczkovski A Jr, Freire GM, Marioni B, Vogt RC, Silveira RD (2012) Mercury concentration in the spectacled caiman and black caiman (alligatoridae) of the amazon: implications for human health. Arch Environ Contam Toxicol 63:270–279
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184:45–58
Chen P, He C (2004) A general strategy to convert the merr family proteins into highly sensitive and selective fluorescent biosensors for metal ions. J Am Chem Soc 126:728–729
Thomas JM, Ting R, Perrin DM (2004) High affinity DNAzyme-based ligands for transition metal cations—a prototype sensor for Hg(II). Org Biomol Chem 2:307
Matsushita M, Meijler MM, Wirsching P, Lerner RA, Janda KD (2005) A blue fluorescent antibody—cofactor sensor for mercury. Cheminform 7:4943–4946
Dirks RM, Pierce NA (2004) From the cover: triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A 101:15275–15278
Chen Y, Xu J, Su J, Xiang Y, Yuan R, Chai YQ (2012) In situ hybridization chain reaction amplification for universal and highly sensitive electrochemiluminescent detection of DNA. Anal Chem 84:7750–7755
Dirks R, Pierce NA (2006) Hybridization chain reaction. Nat Methods 1:186–187
Zhang B, Liu BQ, Tang DP, Niessner R, Chen GN, Knopp D (2012) DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal Chem 84:5392–5399
Liu P, Yang X, Sun S, Wang Q, Wang K, Huang J, Liu J, He L (2013) Enzyme-free colorimetric detection of DNA by using gold nanoparticles and hybridization chain reaction amplification. Anal Chem 85:7689
Bukowska J, Piotrowski P (2014) Surface-enhanced Raman scattering (SERS) in bioscience: a review of application. Springer Netherlands 14:29–59
Mosier-Boss PA (2017) Review of SERS substrates for chemical sensing. Nanomater 7:142
Du Y, Liu R, Liu B, Wang S, Han MY, Zhang Z (2013) Surface-enhanced Raman scattering chip for femtomolar detection of mercuric ion (ii) by ligand exchange. Anal Chem 85:3160–3165
Zhang Y, Zeng G, Tang L, Chen J, Zhu Y, He XX (2015) Electrochemical sensor based on electrodeposited graphene-Au modified electrode and nanoAu carrier amplified signal strategy for attomolar mercury detection. Anal Chem 87:989–996
Koo TW, Chan S, Sun L, Su X, Zhang J, Berlin AA (2004) Specific chemical effects on surface-enhanced Raman spectroscopy for ultra-sensitive detection of biological molecules. Appl Spectrosc 58:1401–1407
Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102
Li M, Zhang J, Suri S, Sooter LJ, Ma D, Wu N (2012) Detection of adenosine triphosphate with an aptamer biosensor based on surface-enhanced Raman scattering. Anal Chem 84:2837–2842
Li CN, Fan PD, Liang AH, Liu QY, Jiang ZL (2012) Aptamer based determination of Pb(II) by SERS and by exploiting the reduction of HAuCl4, by H2O2, as catalyzed by graphene oxide nanoribbons. Microchim Acta 185:177
Zhang CM, Gao YK, Yang N, You TT, Chen HX, Yin PG (2018) Direct determination of the tumor marker AFP via silver nanoparticle enhanced SERS and AFP-modified gold nanoparticles as capturing substrate. Microchim Acta 185:90
Ma PY, Liang FH, Yang QQ, Wang D, Sun Y, Wang XH, Gao DJ, Song DQ (2014) Highly sensitive SERS probe for mercury(II) using cyclodextrin-protected silver nanoparticles functionalized with methimazole. Microchim Acta 181:975–981
Li Y, Qi X, Lei C, Yue Q, Zhang S (2014) Simultaneous SERS detection and imaging of two biomarkers on the cancer cell surface by self-Assembly of branched DNA-gold nanoaggregates. Chem Commun 50:9907–9909
Chen P, Wu P, Zhang Y, Chen J, Jiang X, Zheng C, Hou X (2016) Label-free and separation-free atomic fluorescence spectrometry-based bioassay: sensitive determination of single-strand DNA, protein and double-strand DNA. Anal Chem 88:12386
Verma HN, Singh P, Chavan RM (2014) Gold nanoparticle: synthesis and characterization. Veterinary World 7:72–77
Lee JS, Mirkin CA (2008) Chip-based scanometric detection of mercuric ion using DNA-functionalized gold nanoparticles. Anal Chem 80:6805–6808
Ding C, Zhang Q, Lin JM, Zhang SS (2009) Electrochemical detection of DNA hybridization based on bio-bar code method. Biosens Bioelectron 24:3140–3143
Hu K, Lan D, Li X, Zhang S (2008) Electrochemical DNA biosensor based on nanoporous gold electrode and multifunctional encoded DNA−Au bio bar codes. Anal Chem 80:9124–9130
Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS (2006) Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312:1027–1030
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc 120:1959–1964
Li Y, Chen C, Li B, Sun J, Wang J, Gao Y, Zhao Y, Chai Z (2005) Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. J Anal At Spectrom 21(1):94–96
Hong MQ, Zeng BH, Li MY, Xu XQ, Chen GN (2018) An ultrasensitive conformation-dependent colorimetric probe for the detection of mercury (II) using exonuclease III-assisted target recycling and gold nanoparticles. Microchim Acta 185(1):72
Ravikumar A, Panneerselvam P, Radhakrishnan K (2018) Fluorometric determination of lead(II) and mercury(II) based on their interaction with a complex formed between graphene oxide and a DNAzyme. Microchim Acta 185:2
Li D, Wieckowska A, Willner I (2008) Optical analysis of Hg2+ ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines. Angew Chem 47:3927–3931
Acknowledgments
This work was financially supported by the Fund Project for Shangdong Key R&D Program (2017GGX20121), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1366 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Lv, S., Gong, Y. et al. Sensitive determination of Hg(II) based on a hybridization chain recycling amplification reaction and surface-enhanced Raman scattering on gold nanoparticles. Microchim Acta 185, 363 (2018). https://doi.org/10.1007/s00604-018-2907-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2907-2