Abstract
The authors describe a fluorometric aptamer based assay for adenosine triphosphate (ATP). It is based on the use of carbon dots (CDs) and graphene oxide (GO). The resultant CD-aptamer is adsorbed on the surface of GO via π-stacking and hydrophobic interaction, and the fluorescence of CD-aptamer is quenched via fluorescence resonance energy transfer (FRET) between CDs and GO. If ATP is present, it will bind to the aptamer and the CD-aptamer will be desorbed from GO. This will suppress FRET and the fluorescence of the CDs is restored. Under the optimal conditions and at typical excitation/emission wavelengths of 358/455 nm, the assay has a 80 pM detection limit and a linear range that extends from 0.10 to 5.0 nM concentrations of ATP. The method was successfully applied to the determination of ATP in yogurt samples. This method can also be conceivably applied to the detection of other analytes for which appropriate aptamers are available.

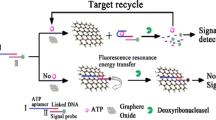
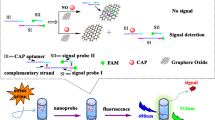
Schematic of a novel fluorometric ATP assay based on the fluorescence resonance energy transfer (FRET) between aptamer modified carbon dots (CD-aptamer) and graphene oxide (GO). CD-aptamer was used as the energy donor and molecular recognition probe, and GO acted as energy acceptor. This assay exhibits high sensitivity and selectivity with a detection limit as low as 80 pM.






Similar content being viewed by others
References
Wang J, Jiang Y, Zhou C, Fang X (2005) Aptamer-based ATP assay using a luminescent light switching complex. Anal Chem 77:3542–3546. https://doi.org/10.1021/ac050165w
Zeng X, Zhang X, Yang W, Jia H, Li Y (2012) Fluorescence detection of adenosine triphosphate through an aptamer-molecular beacon multiple probe. Anal Biochem 424:8–11. https://doi.org/10.1016/j.ab.2012.01.021
Ma C, Yang X, Wang K, Tang Z, Li W, Tan W, Lv X (2008) A novel kinase-based ATP assay using molecular beacon. Anal Biochem 372:131–133. https://doi.org/10.1016/j.ab.2007.08.003
Tang DP, Hou L (2016) Aptasensor for ATP based on analyte-induced dissociation of ferrocene-aptamer conjugates from manganese dioxide nanosheets on a screen-printed carbon electrode. Microchim Acta 183:2705–2711. https://doi.org/10.1007/s00604-016-1916-2
Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA (1999) Glucose generates sub-plasma membrane ATP microdomains in single islet β-cells: potential role for strategically located mitochondria. J Biol Chem 274:13281–13291. https://doi.org/10.1074/jbc.274.19.13281
Chen Z, Li G, Zhang L, Jiang JF, Li Z, Peng ZH, Deng L (2008) A new method for the detection of ATP using a quantum-dot-tagged aptamer. Anal Bioanal Chem 392:1185–1188. https://doi.org/10.1007/s00216-008-2342-z
Gao PY, Xia YF, Yang LL, Ma TF, Yang L, Guo QQ, Huang SS (2014) Aptasensor for adenosine triphosphate based on electrode-supported lipid bilayer membrane. Microchim Acta 181:205–212. https://doi.org/10.1007/s00604-013-1100-x
Zhang SS, Yan YM, Bi S (2009) Design of molecular beacons as signaling probes for adenosine triphosphate detection in cancer cells based on chemiluminescence resonance energy transfer. Anal Chem 81:8695–8701. https://doi.org/10.1021/ac901759g
Cui ZQ, Ren Q, Wei HP, Chen Z, Deng JY, Zhang ZP, Zhang XE (2011) Quantum dot-aptamer nanoprobes for recognizing and labeling influenza a virus particles. Nano 3:2454–2457. https://doi.org/10.1039/c1nr10218d
Lim SY, Shen W, Gao ZQ (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381. https://doi.org/10.1039/C4CS00269E
Citartan M, Gopinath SCB, Tominaga J, Tan SC, Tang TH (2012) Assays for aptamer-based platforms. Biosens bioelectron 34:1–11. https://doi.org/10.1016/j.bios.2012.01.002
Pilehvar S, Mehta J, Dardenne F, Robbens J, Blust R, De Wael K (2012) Aptasensing of chloramphenicol in the presence of its analogues: reaching the maximum residue limit. Anal Chem 84:6753–6758. https://doi.org/10.1021/ac3012522
Feng CJ, Dai S, Wang L (2014) Optical aptasensors for quantitative detection of small biomolecules: a review. Biosens Bioelectron 59:64–74. https://doi.org/10.1016/j.bios.2014.03.014
Chiu TC, Huang CC (2009) Aptamer-functionalized nano-biosensors. Sensors 9:10356–10388. https://doi.org/10.3390/s91210356
Zhang H, Zhang HL, Aldalbahi A, Zuo XL, Fan CH, Mi XQ (2017) Fluorescent biosensors enabled by graphene and graphene oxide. Biosens Bioelectron 89:96–106. https://doi.org/10.1016/j.bios.2016.07.030
Liu MC, Chen CL, Hu J, Wu XL, Wang X (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal. Phys Chem C 115:25234–25240. https://doi.org/10.1021/jp208575m
Loh KP, Bao Q, Eda G, Chhowalla M (2010) Graphene oxide as a chemically tunable platform for optical applications. Nat Chem 2:1015–1024. https://doi.org/10.1038/NCHEM.907
Zhang C, Yuan Y, Zhang S, Wang Y, Liu Z (2011) Biosensing platform based on fluorescence resonance energy transfer from upconverting nanocrystals to graphene oxide. Angew Chem Int Ed 50:6851–6854. https://doi.org/10.1002/anie.201100769
Wu M, Kempaiah R, Huang PJJ, Maheshwari V, Liu JW (2011) Adsorption and desorption of DNA on graphene oxide studied by fluorescently labeled oligonucleotides. Langmuir 27:2731–2738. https://doi.org/10.1021/la1037926
Huang PJJ, Liu J (2012) DNA-length-dependent fluorescence signaling on graphene oxide surface. Small 8:977–983. https://doi.org/10.1002/smll.201102156
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578. https://doi.org/10.1007/s00604-014-1360-0
Zhu SJ, Meng QN, Wang L, Zhang JH, Song YB, Jin H, Zhang K, Sun HC, Wang HY, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem 52:3953–3957. https://doi.org/10.1002/anie.201300519
Zhang LM, Xia JG, Zhao QH, Liu LW, Zhang ZJ (2010) Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6:537–544. https://doi.org/10.1002/smll.200901680
Yan XL, Chen X, Gao WJ, Wei TT, Lin YM, Ai FR (2017) Nanoparticles grafted by ATP aptamer: preparation and application in Chemiluminescence enzyme analysis. Chinese journal of inorganic chemistry 33:340–346. https://doi.org/10.11862/CJIC.2017.034
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Liu T, Dong JX, Liu SG, Li N, Lin SM, Fan YZ, Lei JL, Luo HQ, Li NB (2017) Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of ag/CQDs composite for Hg2+ ions detection. J Hazard Mater 322:430–436. https://doi.org/10.1016/j.jhazmat.2016.10.034
Feng T, Ai XZ, Ong HM, Zhao YL (2016) Dual-responsive carbon dots for tumor extracellular microenvironment triggered targeting and enhanced anticancer drug delivery. ACS Appl Mater Interfaces 8:18732–18740. https://doi.org/10.1021/acsami.6b06695
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2017) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 184:343–368. https://doi.org/10.1007/s00604-016-2043-9
Kudin KN, Ozbas B, Schniepp HC, Prud’Homme RK, Aksay IA, Car R (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8:36–41. https://doi.org/10.1021/nl071822y
Goud KY, Hayat A, Satyanarayana M, Kumar VS, Catanante G, Gobi KV, Marty JL (2017) Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim Acta 184:4401–4408. https://doi.org/10.1007/s00604-017-2487-6
He SB, Huang BH, Tan JJ, Luo QY, Lin Y, Li J, Hu Y, Zhang L, Yan SH, Zhang Q, Pang DW, Li LJ (2011) One-to-one quantum dot-labeled single long DNA probes. Biomaterials 32:5471–5477. https://doi.org/10.1016/j.biomaterials.2011.04.013
Dong HF, Gao WC, Yan F, Ji HX, Ju HX (2010) Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal Chem 82:5511–5517. https://doi.org/10.1021/ac100852z
Liu HY, Xu SM, He ZM, Deng AP, Zhu JJ (2013) Supersandwich Cytosensor for selective and ultrasensitive detection of cancer cells using aptamer-DNA Concatamer-quantum dots probes. Anal Chem 85:3385–3392. https://doi.org/10.1021/ac303789x
Snyder AB, Churey JJ, Worobo RW (2016) Characterization and control of Mucor circinelloides spoilage in yogurt. Int J Food Microbiol 228:14–21. https://doi.org/10.1016/j.ijfoodmicro.2016.04.008
Hilton ST, de Moraes JO, Moraru CI (2017) Effect of sublethal temperatures on pulsed light inactivation of bacteria. Innovative Food Science and Emerging Technologies 39:49–54. https://doi.org/10.1016/j.ifset.2016.11.002
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 61775099, No. 21705080), the Natural Science Foundation of Jiangsu Province (No. BK20171487, No. BK20171043), and the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (No. 17KJB150029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 6687 kb)
Rights and permissions
About this article
Cite this article
Cheng, X., Cen, Y., Xu, G. et al. Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim Acta 185, 144 (2018). https://doi.org/10.1007/s00604-018-2683-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2683-z