Abstract
The authors describe a colorimetric immunoassay for the model nalyte aflatoxin B1 (AFB1). It is based on the just-in-time generation of an MnO2 nanocatalyst. Unlike previously developed immunoassay, the chromogenic reaction relies on the just-in-time formation of an oxidase mimic without the aid of the substrate. Potassium permanganate (KMnO4) is converted into manganese dioxide (MnO2) which acts as an oxidase mimic that catalyzes the oxidation 3,3′,5,5′-tetramethylbenzidine (TMB) by oxygen to give a blue colored product. In the presence of ascorbic acid (AA), KMnO4 is reduced to Mn(II) ions. This results in a decrease in the amount of MnO2 nanocatalyst. Hence, the oxidation of TMB does not take place. By adding ascorbate oxidase, AA is converted into dehydroascorbic acid which cannot reduce KMnO4. Based on these observations, a colorimetric competitive enzyme immunoassay was developed where ascorbate oxidase and gold nanoparticle-labeled antibody against AFB1 and magnetic beads carrying bovine serum albumin conjugated to AFB1 are used for the determination of AFB1. In presence of AFB1, it will compete with the BSA-conjugated AFB1 (on the magnetic beads) for the labeled antibody against AFB1 on the gold nanoparticles. This makes the amount of ascorbate oxidase/anti-AFB1 antibody-labeled gold nanoparticles, which conjugated on magnetic beads, reduce, and resulted in an increase of ascorbic acid. Under optimal conditions, the absorbance (measured at 652 nm) decreases with increasing AFB1 concentrations in the range from 0.1 to 100 ng mL−1, with a 0.1 ng mL−1 detection limit (at the 3Sblank level). The accuracy of the assay was validated by analyzing spiked peanut samples. The results matched well with those obtained with a commercial ELISA kit. Conceivably, the method is not limited to aflatoxins but has a wide scope in that it may be applied to many other analytes for which respective antibodies are available.

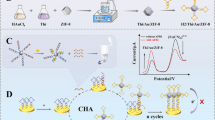
Schematic illustration of ascorbate oxidase (AOx)-mediated potassium permanganate (KMnO4)-responsive ascorbic acid (AA) for visual colorimetric immunoassay of aflatoxin B1 (AFB1) by coupling with hydrolytic reaction of AOx toward AA and the KMnO4-Mn(II)-TMB system [note: 3,3′,5,5′-tetramethylbenzidine: TMB].





Similar content being viewed by others
References
Abbas H, Accinelli C, Shier W (2017) Biological control of aflatoxin contamination in U.S. crops and the use of bioplastic formulations of aspergillus flavus biocontrol strains to optimize application strategies. J Agric Food Chem 65:7081–7087
Qi D, Fei T, Liu H, Yao H, Wu D, Liu B (2017) Development of multiple heart-cutting two-dimensional liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry for simultaneous determination of Aflatoxin B1, B2, G1, G2, and ochratoxin A in snus, a smokeless tobacco product. J Agric Food Chem 65:9923–9929
Lim C, Yomoya T, Layne J, Chan S (2015) Multi-mycotoxin screening reveals separate occurrence of aflatoxins and ochratoxin A in Asian rice. J Agric Food Chem 63:3104–3113
Li X, Yang F, Wong J, Yu H (2017) Integrated smartphone-app-chip system for on-site parts-per-billion-level colorimetric quantitation of aflatoxins. Anal Chem 89:8908–8916
Du B, Su X, Yang K, Pan L, Liu Q, Gong L, Wang P, Yang J, He Y (2017) Antibody-free colorimetric detection of total aflatoxins in rice based on a simple two-step chromogenic reaction. Anal Chem 89:4809–4815
Xie J, Jiang H, Shen J, Peng T, Wang J, Yao K, Sun S, Shao B, Tang J (2017) Design of multifunctional nanostructure for ultrafast extraction and purification of aflatoxins in foodstuffs. Anal Chem 89:10556–10564
Zitomer N, Rybak M, Li Z, Walters M, Holman M (2015) Determination of aflatoxin B1 in smokeless tobacco products by use of UHPLC-MS/MS. J Agric Food Chem 63:9131–9138
Eltzov E, Marks R (2017) Colorimetric stack pad immunoassay for bacterial identification. Biosens Bioelectron 87:572–578
Gao Z, Xu M, Hou L, Chen G, Tang D (2017) High-index {hk0} faceted platinum concave nanocubes with enhanced peroxidase-like activity for an ultrasensitive colorimetric immunoassay of the human prostate-specific antigen. Analyst 142:911–917
Lai W, Wei X, Zhuang J, Lu M, Tang D (2016) Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens Bioelectron 80:249–256
Fu X, Chen L, Choo J (2017) Optical nanoprobes for ultrasensitive immunoassay. Anal Chem 89:124–137
Liu Y, Zhang L, Wei W, Zhao H, Zhao Z, Zhang Y, Liu S (2015) Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst 140:3989–3995
Xu S, Ouyang W, Xie P, Lin Y, Qiu B, Lin Z, Chen G, Guo L (2017) Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal Chem 89:1617–1623
Ma X, Chen Z, Kannan P, Lin Z, Qiu B, Guo L (2016) Gold nanorods as colorful chromogenic substrates for semiquantitative detection of nucleic acids, proteins, and small molecules with the naked eye. Anal Chem 88:3227–3234
Lai W, Zhuang J, Tang D (2015) Novel colorimetric immunoassay for ultrasensitive monitoring of brevetoxin B based on enzyme-controlled chemical conversion of sulfite to sulfate. J Agric Food Chem 63:1982–1989
Gao Z, Xu M, Hou L, Chen G, Tang D (2013) Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal Chim Acta 776:79–86
Liu M, Jia C, Jin Q, Lou X, Yao S, Xiang J, Zhao J (2010) Novel colorimetric enzyme immunoassay for the detection of carcinoembryonic antigen. Talanta 81:1625–1629
Gao Z, Hou L, Xu M, Tang D (2014) Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci Rep 4:3966
Hu Y, Cheng H, Zhao X, Wu J, Muhammad F, Lin S, He J, Zhou L, Zhang C, Deng Y, Wang P, Zhou Z, Nie S, Wei H (2017) Surface-enhanced raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 11:5558–5566
Lai W, Wei Q, Xu M, Zhuang J, Tang D (2017) Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens Bioelectron 89:645–651
Li H, Liu H, Zhang J, Cheng Y, Zhang C, Fei X, Xian Y (2017) Platinum nanoparticle encapsulated metal-organic frameworks for colorimetric measurement and facile removal of mercury(II). ACS Appl Mater Interfaces 9:40716–40725
Zhang C, Tang J, Huang L, Li Y, Tang D (2017) In-situ amplified voltammetric immunoassay for ochratoxin A by coupling a platinum nanocatalyst based enhancement to a redox cycling process promoted by an enzyme mimic. Microchim Acta 184:2445–2453
Yan X, Song Y, Wu X, Zhu C, Su X, Du D, Lin Y (2017) Oxidase-mimicking activity of ultrathin MnO2 nanosheets in colorimetric assay of acetylcholinesterase activity. Nano 9:2317–2323
Pal J, Pal T (2016) Enzyme mimicking inorganic hybrid Ni@MnO2 for colorimetric detection of uric acid in serum samples. RSC Adv 6:83738–83747
Lin L, Shi D, Li Q, Wang G, Zhang X (2016) Detection of T4 polynucleotide kinase based on a MnO2 nanosheet-3,3′,5,5′-tetramethylbenzidine (TMB) colorimetric system. Anal Methods 8:4119–4126
Zhai W, Wang C, Yu P, Wang Y, Mao L (2014) Single-layer MnO2 nanosheets suppressed fluorescence of 7-hydroxycoumarin: mechanistic study and application for sensitive sensing of ascorbic acid in vivo. Anal Chem 86:12206–12213
European Commission (2006) Regulation (EC) No. 1881/2006: Off. J. Eur. Union, 255, 14–17
Tang Y, Lai W, Zhang J, Tang D (2017) Competitive photometric and visual ELISA for aflatoxin B1 based on the inhibition of the oxidation of ABTS. Microchim Acta 184:2387–2394
Acknowledgements
Support by the National Natural Science Foundation of China (Grants No. 21505060), the Outstanding Youth Science Foundation of Fujian Province (Year 2017), the Program for Excellent Talents of Minnan Normal University (Grant No. MJ1601), the Natural Science Foundation of Zhangzhou City, China (Grant No. ZZ2016J30), the National Science Foundation of Fujian Province (Grant No. 2014 J07001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 403 kb)
Rights and permissions
About this article
Cite this article
Lai, W., Zeng, Q., Tang, J. et al. A conventional chemical reaction for use in an unconventional assay: A colorimetric immunoassay for aflatoxin B1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst. Microchim Acta 185, 92 (2018). https://doi.org/10.1007/s00604-017-2651-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2651-z