Abstracts
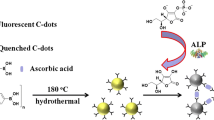
Boron and nitrogen codoped carbon dots functionalized with cyclodextrin (β-CD-N/B-C-dots) were obtained from β-cyclodextrin. The material displays strong fluorescence (with excitation/emission peak wavelengths of 400/500 nm) and was characterized by UV-vis, transmission electron microscopy and FTIR. If the substrate p-nitrophenylphosphate is enzymatically cleaved by alkaline phosphatase (ALP), a yellow product is formed whose absorption overlaps the excitation spectrum of the β-CD-N/B-C-dots. Hence, fluorescence is reduced due to an inner filter effect. In additon, the β-CD cavity offers a pocket for substrate recognition. The findings were used to design a method for the determination of the activity of ALP. It has a working range that extends from 0.003 to 5.5 U·L−1, with a 0.3 mU·L−1 detection limit. The method is fast, simple, inexpensive, and highly sensitive and selective.

Schematic of an inner filter effect based probe for alkaline phosphatase based on the use boron and nitrogen co-doped carbon dots (N/B-C-dots) modified with β-cyclodextrin (β-CD). PNPP: p-Nitrophenylphosphate; PNP: p-Nitrophenol anion.





Similar content being viewed by others
References
Harris H (1990) The human alkaline phosphatases: what we know and what we don't know. Clin Chim Acta 186:133
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441
Fernley H (1971) 18 mammalian alkaline phosphatases. Enzyme 4:417
Zhao MM, Guo YJ, Wang LX, Luo F, Lin CY, Lin ZY, Chen GN (2016) A sensitive fluorescence biosensor for alkaline phosphatase activity based on the Cu(II)-dependent DNAzyme. Anal Chim Acta 948:98
Lorente JA, Valenzuela H, Morote J, Gelabert A (1999) Serum bone alkaline phosphatase levels enhance the clinical utility of prostate specific antigen in the staging of newly diagnosed prostate cancer patients. Eur J Nucl Med 26:625
Bricon TL, Gay-Bellile C, Cottu P, Benlakehal M, Guillon H, Houze P (2010) Lectin affinity electrophoresis of serum alkaline phosphatase in metastasized breast cancer. J Clin Lab Anal 24:20
Miller PD (2014) Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis 64:290
Limdi JK, Hyde GM (2003) Evaluation of abnormal liver function tests. Postgrad Med J 79:307
Lassenius MI, Fogarty CL, Blaut M, Haimila K, Riittinen L, Paju A, Kirveskari J (2017) Intestinal alkaline phosphatase at the crossroad of intestinal health and disease - a putative role in type 1 diabetes. J Intern Med 281:586
Yang JJ, Zheng L, Wang Y, Li W, Zhang JL, JJ G, Fu Y (2016) Guanine-rich DNA-based peroxidase mimetics for colorimetric assays of alkaline phosphatase. Biosens Bioelectron 77:549
Hu Q, Zhou BJ, Dang PY, Li LZ, Kong JM, Zhang XJ (2017) Facile colorimetric assay of alkaline phosphatase activity using Fe(II)-phenanthroline reporter. Anal Chim Acta 950:170
Hu Q, He MH, Mei YQ, Feng WJ, Jing S, Kong JM, Zhang XJ (2017) Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu(II)-phenanthroline complex. Talanta 163:146
Ingram A, Moore BD, Graham D (2009) Simultaneous detection of alkaline phosphatase and β-galactosidase activity using SERRS. Bioorg Med Chem Lett 19:1569
Ruan CM, Wang W, BH G (2006) Detection of alkaline phosphatase using surface-enhanced Raman spectroscopy. Anal Chem 78:3379
Xiang MH, Liu JW, Li N, Tang H, RQ Y, Jiang JH (2016) A fluorescent graphitic carbon nitride nanosheet biosensor for highly sensitive, label-free detection of alkaline phosphatase. Nano 8:4727
Deng J, Yu P, Wang Y, Mao L (2015) Real-time ratiometric fluorescent assay for alkaline phosphatase activity with stimulus responsive infinite coordination polymer nanoparticles. Anal Chem 87:3080
Kong RM, Fu T, Sun NN, FL Q, Zhang SF, Zhang XB (2013) Pyrophosphate-regulated Zn2+-dependent DNAzyme activity: an amplified fluorescence sensing strategy for alkaline phosphatase. Biosens Bioelectron 50:351
Zhang L, Hou T, Li H, Li F (2015) A highly sensitive homogeneous electrochemical assay for alkaline phosphatase activity based on single molecular beaconinitiated T7 exonuclease-mediated signal amplification. Analyst 140:4030
Zhang HM, CL X, Liu J, Li XH, Guo L, Li XM (2015) An enzyme-Activatable probe with a self-Immolative linker for rapid and sensitive alkaline phosphatase detection and cell imaging through a Cascade reaction. Chem Commun 51:7031
Chen J, Jiao HP, Li WY, Liao DL, Zhou HP, Yu C (2013) Real-time fluorescence turn-on detection of alkaline phosphatase activity with a novel Perylene probe. Chem-Asian J 8:276
Liu Y, Schanze KS (2008) Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal Chem 80:8605
Liu H, Lv ZL, Ding KG, Liu XL, Yuan L, Chen H, Li XM (2013) Incorporation of tyrosine phosphate into tetraphenylethylene affords an amphiphilic molecule for alkaline phosphatase detection, hydrogelation and calcium mineralization. J Mater Chem B 1:5550
Liu SY, Pang S, Na WD, XG S (2014) Near-infrared fluorescence probe for the determination of alkaline phosphatase. Biosens Bioelectron 55:249
Jia L, JP X, Li D, Pang SP, Fang YA, Song ZG, Ji JA (2010) Fluorescence detection of alkaline phosphatase activity with Beta-Cyclodextrin-modified quantum dots. Chem Commun 46:7166
Lim SY, Shen W, Gao ZQ (2015) Carbon quantum dots and their applications. Chem Soc Rev 449:362
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11:1620
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8:355
Yang Y, Zhang JC, Zhuang J, Wang X (2015) Synthesis of nitrogen-doped carbon nanostructures from polyurethane sponge for bioimaging and catalysis. Nano 7:12284
Hu C, Yu C, Li MY, Wang XN, Yang JY, Zhao ZB, Eychmuller A, Sun YP, Qiu JS (2014) Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu(II) detection. Small 10:4926
Tian T, He Y, Ge YL, Song GW (2017) One-pot synthesis of boron and nitrogen co-doped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr(VI) and dopamine. Sensors Actuators B Chem 240:1265
Wenz G, Han BH, Muller A (2006) Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev 106:782
Zhao XH, Liu X, Lu M (2014) β-cyclodextrin-capped palladium nanoparticle-catalyzed ligand-free Suzuki and Heck couplings in low-melting β-cyclodextrin/NMU mixtures. Appl Organomet Chem 28:635
Putta C, Sharavath V, Sarkar S, Ghosh S (2014) Palladium nanoparticles on β-cyclodextrin functionalised graphene nanosheets: a supramolecular based heterogeneous catalyst for C–C coupling reactions under green reaction conditions. RSC Adv 5:6652
Chen S, Zhang JB, Gan N, FT H, Li TH, Cao YT, Pan DD (2015) An on-site immunosensor for ractopamine based on a personal glucosemeter and using magnetic β-cyclodextrin-coated nanoparticles for enrichment, and an invertase-labeled nanogold probe for signal amplification. Microchim Acta 182:815
Tang C, Qian ZS, Huang YY, JM X, Ao H, Zhao MZ, Zhou J, Chen JR, Feng H (2016) A fluorometric assay for alkaline phosphatase activity based on β-cyclodextrin-modified carbon quantum dots through host-guest recognition. Biosens Bioelectron 83:274
Liu H, Ma C, Wang J, Wang K, Wu K (2017) A turn-on fluorescent method for determination of the activity of alkaline phosphatase based on dsDNA-templated copper nanoparticles and exonuclease based amplification. Microchim Acta 184:2483
Kang W, Ding Y, Zhou H, Liao Q, Yang X, Yang Y, Yang M (2015) Monitoring the activity and inhibition of alkaline phosphatase via quenching and restoration of the fluorescence of carbon dots. Microchim Acta 182:1161
Guo L, Chen D, Yang M (2017) DNA-templated silver nanoclusters for fluorometric determination of the activity and inhibition of alkaline phosphatase. Microchim Acta 184:2165
Liu XG, Xing XJ, Li B, Guo YM, Zhang YZ, Yang Y, Zhang LF (2016) Fluorescent assay for alkaline phosphatase activity based on graphene oxide integrating with λ exonuclease. Biosens Bioelectron 81:460
Wang Y, Chen J, Jiao H, Chen Y, Li W, Zhang Q, Yu C (2013) Polymer templated perylene-probe noncovalent self-assembly: a new strategy for label-free ultrasensitive fluorescence turn-on biosensing. Chem Eur J 19:12846
Li GL, HL F, Chen XJ, Gong PW, Chen G, Xia L, Wang H, You JM, YN W (2016) Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal Chem 88:2720
Liu JJ, Wang J, Zhu ZM, Li L, Guo XH (2014) Cooperative catalytic activity of Cyclodextrin and ag nanoparticles immobilized on spherical polyelectrolyte brushes. AICHE J 60:1977
Acknowledgements
This work was financially supported by Supported by National Natural Science Foundation of China (21707030) and Wuhan Youth Science and technology plan (2016070204010133).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 234 kb)
Rights and permissions
About this article
Cite this article
Mao, M., Tian, T., He, Y. et al. Inner filter effect based fluorometric determination of the activity of alkaline phosphatase by using carbon dots codoped with boron and nitrogen. Microchim Acta 185, 17 (2018). https://doi.org/10.1007/s00604-017-2541-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2541-4