Abstract
We report on a stable and uniform nanocomposite (NC) as an enhancing element on the surface of a screen printed electrode for the detection of the M268T mutation of Angiotensinogen gene by DNA hybridization and DNA synthesis. This NC consists of multiwalled carbon nanotubes, pyrenebutyric acid (PBA) and chitosan. Two kinds of DNA biosensors were constructed by covalently coupling amino modified oligonucleotides to the carboxylic groups of PBA. Methylene Blue (MB) was employed as an electroactive probe for the detection of DNA. The two DNA biosensors were applied to detect the complementary sequence by differential pulse voltammetry. The results suggested that the peak currents of MB on the two biosensors are linearly related to the logarithm of the concentrations of target DNA in the 1.0 aM to 10 nM and in the 1 aM to 0.1 nM ranges, with detection limits of 0.11 and 0.24 aM for normal and mutant DNA, respectively. The selectivity experiment also showed the biosensors to be able to distinguish between target DNA and non-complementary sequences, and between normal homozygote, mutant homozygote and heterozygote. The biosensor was applied to quantify the products of PCR amplification of the Angiotensinogen gene (that is related to Atherosclerosis) extracted from human blood samples and gave satisfactory results. We expect this scheme to possess potential application in the detection of other genes.

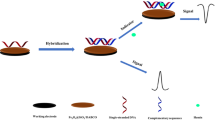
A screen-printed electrode (SPE) was modified with a nanocomposite in order to detect the M268T angiotensin mutation gene by an electrochemical method.






Similar content being viewed by others
References
Lusis AJ (2012) Genetics of atherosclerosis. Trends Genet 28:267–275
Mak S, Sun H, Acevedo F et al (2010) Differential expression of genes in the calcium-signaling pathway underlies lesion development in the {LDb} mouse model of atherosclerosis. Atherosclerosis 213:40–51
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2014) Screen-printed electrodes for biosensing: a review (2008–2013). Microchim Acta 161:865–891
He L, Zhang Y, Liu S et al (2014) A nanocomposite consisting of plasma-polymerized propargylamine and graphene for use in DNA sensing. Microchim Acta 181:1981–1989
Mazloum-Ardakani M, Ahmadi R, Heidari MM, Sheikh-Mohseni MA (2014) Electrochemical detection of the MT-ND6 gene and its enzymatic digestion: application in human genomic sample. Anal Biochem 455:60–64
Mazloum-Ardakani M, Khoshroo A, Hosseinzadeh L (2014) Application of graphene to modified ionic liquid graphite composite and its enhanced electrochemical catalysis properties for levodopa oxidation. Sensors Actuators B Chem 204:282–288
Mazloum-Ardakani M, Khoshroo A (2014) Electrocatalytic properties of functionalized carbon nanotubes with titanium dioxide and benzofuran derivative/ionic liquid for simultaneous determination of isoproterenol and serotonin. Electrochim Acta 130:634–641
Mazloum-Ardakani M, Khoshroo A (2014) High sensitive sensor based on functionalized carbon nanotube/ionic liquid nanocomposite for simultaneous determination of norepinephrine and serotonin. J Electroanal Chem 717–718:17–23
Mazloum-Ardakani M, Hosseinzadeh L, Taleat Z (2014) Two kinds of electrochemical immunoassays for the tumor necrosis factor α in human serum using screen-printed graphite electrodes modified with poly (anthranilic acid). Microchim Acta 161:917–924
Zhang Y, Zhang K, Ma H (2009) Electrochemical {DNA} biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal Biochem 387:13–19
Cao X (2014) Ultra-sensitive electrochemical DNA biosensor based on signal amplification using gold nanoparticles modified with molybdenum disulfide, graphene and horseradish peroxidase. Microchim Acta 181:1133–1141
Mazloum-Ardakani M, Khoshroo A (2013) Nano composite system based on coumarin derivative–titanium dioxide nanoparticles and ionic liquid: determination of levodopa and carbidopa in human serum and pharmaceutical formulations. Anal Chim Acta 798:25–32
Mazloum-Ardakani M, Khoshroo A (2014) High performance electrochemical sensor based on fullerene-functionalized carbon nanotubes/Ionic liquid: determination of some catecholamines. Electrochem Commun 42:9–12
Yang X, Feng B, He X et al (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180:935–956
Zhang M, Smith A, Gorski W (2004) Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem 76:5045–5050
Zhao G, Zhan X (2010) Facile preparation of disposable immunosensor for Shigella flexneri based on multi-wall carbon nanotubes/chitosan composite. Electrochim Acta 55:2466–2471
García-González R, Costa-García A, Fernández-Abedul MT (2014) Methylene blue covalently attached to single stranded {DNA} as electroactive label for potential bioassays. Sensors Actuators B Chem 191:784–790
Sun W, Zhang Y, Ju X et al (2012) Electrochemical deoxyribonucleic acid biosensor based on carboxyl functionalized graphene oxide and poly-l-lysine modified electrode for the detection of tlh gene sequence related to vibrio parahaemolyticus. Anal Chim Acta 752:39–44
Wang Y, Zhou A (2007) Spectroscopic studies on the binding of methylene blue with DNA by means of cyclodextrin supramolecular systems. J Photochem Photobiol A Chem 190:121–127
Pan D, Zuo X, Wan Y et al (2007) Electrochemical interrogation of interactions between surface-confined DNA and methylene blue. Sensors 7:2671–2680
Farjami E, Clima L, Gothelf KV, Ferapontova EE (2010) DNA interactions with a methylene blue redox indicator depend on the DNA length and are sequence specific. Analyst 135:1443–1448
Rohs R, Sklenar H, Lavery R, Röder B (2000) Methylene blue binding to DNA with alternating GC base sequence: a modeling study. J Am Chem Soc 122:2860–2866
Lubin AA, Lai RY, Baker BR et al (2006) Sequence-specific, electronic detection of oligonucleotides in blood, soil, and foodstuffs with the reagentless, reusable E-DNA sensor. Anal Chem 78:5671–5677
Pänke O, Kirbs A, Lisdat F (2007) Voltammetric detection of single base-pair mismatches and quantification of label-free target ssDNA using a competitive binding assay. Biosens Bioelectron 22:2656–2662
Ozkan D, Kara P, Kerman K et al (2002) {DNA} and {PNA} sensing on mercury and carbon electrodes by using methylene blue as an electrochemical label. Bioelectrochemistry 58:119–126
Gao H, Qi X, Chen Y, Sun W (2011) Electrochemical deoxyribonucleic acid biosensor based on the self-assembly film with nanogold decorated on ionic liquid modified carbon paste electrode. Anal Chim Acta 704:133–138
Katz E, Willner I (2004) Biomolecule‐functionalized carbon nanotubes: applications in nanobioelectronics. ChemPhysChem 5:1084–1104
Meng L, Fu C, Lu Q (2009) Advanced technology for functionalization of carbon nanotubes. Prog Nat Sci 19:801–810
McQueen EW, Goldsmith JI (2009) Electrochemical analysis of single-walled carbon nanotubes functionalized with pyrene-pendant transition metal complexes. J Am Chem Soc 131:17554–17556
Chen RJ, Zhang Y, Wang D, Dai H (2001) Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J Am Chem Soc 123:3838–3839
Qian L, Yang X (2006) Composite film of carbon nanotubes and chitosan for preparation of amperometric hydrogen peroxide biosensor. Talanta 68:721–727
Truong LTN, Chikae M, Ukita Y, Takamura Y (2011) Labelless impedance immunosensor based on polypyrrole – pyrolecarboxylic acid copolymer for hCG detection. Talanta 85:2576–2580
Garcinuño B, Ojeda I, Moreno-Guzmán M et al (2014) Amperometric immunosensor for the determination of ceruloplasmin in human serum and urine based on covalent binding to carbon nanotubes-modified screen-printed electrodes. Talanta 118:61–67
Rodríguez MC, Rivas GA (2009) Label-free electrochemical aptasensor for the detection of lysozyme. Talanta 78:212–216
Kirby R, Cho EJ, Gehrke B et al (2004) Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal Chem 76:4066–4075
Primrose SB, Saunders GC, Parkes HC (1999) Analytical molecular biology: quality and validation. Royal Soc Chem
Zhang X, Gao F, Cai X et al (2013) Application of graphene–pyrenebutyric acid nanocomposite as probe oligonucleotide immobilization platform in a {DNA} biosensor. Mater Sci Eng C 33:3851–3857
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Liu Y, Zhou Q, Revzin A (2013) An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 138:4321–4326
Benvidi A, Rajabzadeh N, Zahedi HM et al (2015) Simple and label-free detection of DNA hybridization on a modified graphene nanosheets electrode. Talanta 137:80–86
Acknowledgments
The authors wish to thank the Iran National Science Foundation (INSF), Yazd University Research Council and the IUT Research Council and Excellence in Sensors for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2082 kb)
Rights and permissions
About this article
Cite this article
Mazloum-Ardakani, M., Hosseinzadeh, L. & Heidari, M.M. Detection of the M268T Angiotensinogen A3B2 mutation gene based on screen-printed electrodes modified with a nanocomposite: application to human genomic samples. Microchim Acta 183, 219–227 (2016). https://doi.org/10.1007/s00604-015-1616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1616-3