Abstract
A novel type of porous metal-organic framework (MOF) was obtained from thiol-modified silica nanoparticles and the copper(II) complex of trimesic acid. It is shown that this nanocomposite is well suitable for the preconcentration of Hg(II) ions. The nanocomposite was characterized by Fourier transfer infrared spectroscopy, X-ray powder diffraction, energy-dispersive X-ray diffraction and scanning electron microscopy. The effects of pH value, sorption time, elution time, the volume and concentration of eluent were investigated. Equilibrium isotherms were studied, and four models were applied to analyze the equilibrium adsorption data. The results revealed that the adsorption process obeyed the Langmuir model. The maximum monolayer capacity and the Langmuir constant are 210 mg g−1 and 0.273 L mg−1, respectively. The new MOF-based nanocomposite is shown to be an efficient and selective sorbent for Hg(II). Under the optimal conditions, the limit of detection is 20 pg mL−1 of Hg(II), and the relative standard deviation is <7.2 % (for n = 3). The sorbent was successfully applied to the rapid extraction of Hg(II) ions from fish, sediment, and water samples.

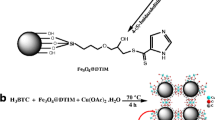
Schematic illustration of Hg(II) sorption onto SH@SiO2/MOF nanocomposite.




Similar content being viewed by others
References
Idris AS, Harvey SR, Gibson LT (2011) Selective extraction of mercury(II) from water samples using mercapto functionalised-MCM-41 and regeneration of the sorbent using microwave digestion. J Hazard Mater 193:171–176
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831–836
Cossa D, Sanjuan J, Cloud J, Stockwell PB, Toms WT (1995) Automated technique for mercury determination at sub-nanogram per litre levels in natural waters. J Anal Spectrom 10:287–291
Dakova I, Karadjova I, Georgieva V, Georgiev G (2009) Ion-imprinted polymethacrylic microbeads as new sorbent for preconcentration and speciation of mercury. Talanta 78:523–529
Zhai Y, Duan S, He Q, Yang X, Han Q (2010) Solid phase extraction and preconcentration of trace mercury(II) from aqueous solution using magnetic nanoparticles doped with 1,5-diphenylcarbazide. Microchim Acta 169:353–360
Li G, Zhao Z, Liu J, Jiang G (2011) Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica. J Hazard Mater 192:277–283
Tuzen M, Soylak M (2005) Mercury contamination in mushroom samples from Tokat, Turkey. Bull Environ Contam Toxicol 74:968–972
Baghdadi M, Shemirani F (2008) Cold-induced aggregation microextraction: a novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bryce DW, Izquierdo A, Luque de Castro MD (1996) Continuous microwave assisted pervaporation/atomic fluorescence detection: an approach for speciation in solid samples. Anal Chim Acta 324:69–75
de Wuilloud JCA, Wuilloud RG, Silva MF, Olsina RA, Martinez LD (2002) Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry. Spectrochim Acta Part B 57:365–374
Chen J, Chen H, Jin X, Chen H (2009) Determination of ultra-trace amount methyl-, phenyl- and inorganic mercury in environmental and biological samples by liquid chromatography with inductively coupled plasma mass spectrometry after cloud point extraction preconcentration. Talanta 77:1381–1387
Karadjova I, Arpadjan S, Cvetkovic J, Stafilov T (2004) Sensitive method for trace determination of Mercury in wines using electrothermal atomic absorption spectrometry. Microchim Acta 147:39–43
Leyva D, Esteˇıvez J, Montero A, Pupo I (2007) Sub-ppm determination of Hg and Cr in water: Cr speciation. X-Ray Spectrom 36:355–360
Hosseini-Bandegharaei A, Hosseini MS, Jalalabadi Y, Sarwghadi M, Nedaie M, Taherian A, Ghaznavi A, Eftekhari A (2011) Removal of Hg(II) from aqueous solutions using a novel impregnated resin containing 1-(2-thiazolylazo)-2-naphthol (TAN). Chem Eng J 168:1163–1173
Horvat M, Liang L, Bloom NS (1993) Comparison of distillation with other current isolation methods for the determination of methyl mercury compounds in low level environmental samples, Part II: water. Anal Chim Acta 282:153–168
Bic N, Sungur S, Gazi M, Tan N (2003) Selective liquid–liquid extraction of Mercuric ions by octyl methane sulfonamide. Sep Sci Technol 38:201–217
Doula MK (2009) Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Res 43:3659–3672
Ebrahimzadeh H, Asgharinezhad AA, Tavassoli N, Sadeghi O, Amini MM, Kamarei F (2012) Separation and spectrophotometric determination of very low levels of Cr(VI) in water samples by novel pyridine-functionalized mesoporous silica. Int J Environ Anal Chem 92:509–521
Sepehrian H, Waqif-Husain S, Ghannadi-Maragheh M (2009) Development of thiolfunctionalized mesoporous silicate MCM-41 as a modified sorbent and its use in chromatographic separation of metal ions from aqueous nuclear waste. Chromatographia 70:277–280
Liu AM, Hidajat K, Kawi S, Zhao DY (2000) A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun 1145–1146
Vieira EFS, Simoni JD, Airoldi C (1997) Interaction of cations with SH-modified silica gel: thermochemical study through calorimetric titration and direct extent of reaction determination. J Mater Chem 7:2249–2252
Ke F, Qiu L-G, Yuan Y-P, Peng F-M, Jiang X, Xie A-J, Shen Y-H, Zhu J-F (2011) Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J Hazard Mater 196:36–43
Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT (2009) Metal-organic framework materials as catalysts. Chem Soc Rev 38:1450–1459
Corma A, García H, Llabrés i Xamena FX (2010) Engineering metal organic frameworks for heterogeneous catalysis. Chem Rev 110:4606–4655
Sohrabi MR, Matbouie Z, Asgharinezhad AA, Dehghani A (2013) Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim Acta 180:589–597
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang J-S, Hwang YK, Marsaud V, Bories P-N, Cynober L, Gil S, Férey G, Couvreur P, Gref R (2010) Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater 9:172–178
Hu YH, Zhang L (2010) Hydrogen storage in metal-organic frameworks. Adv Mater 22:E117–E130
Qiu LG, Li ZQ, Wu Y, Wang W, Xu T, Jiang X (2008) Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of organoamines. Chem Commun 3642–3644
Koh K, Wong-Foy AG, Matzger AJ (2009) A porous coordination copolymer with over 5000 m2/g BET surface area. J Am Chem Soc 131:4184–4185
Wang ZQ, Cohen SM (2009) Postsynthetic modification of metal-organic frameworks. Chem Soc Rev 38:1315–1329
Ke F, Yuan Y-P, Qiu L-G, Shen Y-H, Xie A-J, Zhu J-F, Tianc X-Y, Zhang L-D (2011) Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J Mater Chem 21:3843–3848
Yilmaz AB (2003) Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay. Turk Environ Res 92:277–281
Ebrahimzadeh H, Molaei K, Asgharinezhad AA, Shekari N, Dehghani Z (2013) Molecularly imprinted nano particles combined with miniaturized homogenous liquid-liquid extraction for the selective extraction of loratadine in plasma and urine samples followed by HPLC-PDA detection. Anal Chim Acta 767:155–162
Aharoni A, Ungarish M (1972) Kinetics of activated chemisorptions-part 2. Theoretical models. J Chem Soc Faraday Trans 73:456–464
Huston ND, Yang RT (1997) Theoretical basis for the Dubinin–Radushkevitch (D–R) adsorption isotherm equation. Adsorption 3:189–195
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloid Surf A 177:169–181
Zhai Y, Chang X, Cui Y, Lia N, Lai S (2006) Selective determination of trace mercury(II) after preconcentration with 4-(2-pyridylazo)-resorcinol-modified nanometer-sized SiO2 particles from sample solutions. Microchim Acta 154:253–259
Acknowledgments
The author would like to thank for the financial support from North Tehran Branch, Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohrabi, M.R. Preconcentration of mercury(II) using a thiol-functionalized metal-organic framework nanocomposite as a sorbent. Microchim Acta 181, 435–444 (2014). https://doi.org/10.1007/s00604-013-1133-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1133-1