Abstract
Aims
Neonatal diabetes mellitus (NDM) is a rare disease where diabetes presents during the first six months of life. There are two types of this disorder: permanent neonatal diabetes (PNDM) and transient neonatal diabetes mellitus (TNDM). PNDM occurs due to mutations in genes involved in either beta-cell survival, insulin regulation, and secretion. This study aims to define the genetic aetiology and clinical phenotypes of PNDM in a large Egyptian cohort from a single centre.
Methods
Patients with PNDM who were diagnosed, treated, or referred for follow-up between January 2002 and January 2021 were identified and clinically phenotyped. All patients were tested for mutations in EIF2AK3, KCNJ11, ABCC8, INS, FOXP3, GATA4, GATA6, GCK, GLIS3, HNF1B, IER3IP1, PDX1, PTF1A, NEUROD1, NEUROG3, NKX2-2, RFX6, SLC2A2, SLC19A2, STAT3, WFS1, ZFP57 using targeted next-generation sequencing (NGS) panel. INSR gene mutation was tested in one patient who showed clinical features of insulin resistance.
Results
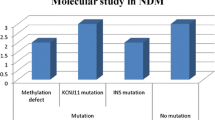
Twenty-nine patients from twenty-six families were diagnosed with PNDM. Pathogenic variants were identified in 17/29 patients (59%). EIF2AK3, INS, and KATP channel mutations were the commonest causes with frequency of 17%, 17%, and 14%, respectively. Patients with ABBC8 and KCNJ11 mutations were successfully shifted to sulfonylureas (SU). Paired data of glycosylated haemoglobin before and after SU transfer showed improved glycaemic control; 9.6% versus 7.1%, P = 0.041.
Conclusions
PNDM is a heterogenous disease with variable genotypes and clinical phenotypes among Egyptian patients. EIF2AK3, INS, ABCC8, and KCNJ11 mutations were the commonest causes of PNDM in the study cohort. All patients with KATP channel mutations were effectively treated with glyburide, reflecting the fact that genetic testing for patients with NDM is not only important for diagnosis but also for treatment plan and prognosis.


Similar content being viewed by others
Data availability
All patients’ data are available in Table 2. Any additional materials are available from the corresponding author on reasonable request.
Code availability
The clinical data were analysed using the Statistical Package of Social Science (SPSS) programme for Windows (Standard version 21).
References
Rabbone I, Barbetti F, Gentilella R et al (2017) Insulin therapy in neonatal diabetes mellitus: a review of the literature. Diabetes Res Clin Pract 129:126–135
Hattersley AT, Greeley S, Polak M et al (2018) ISPAD clinical practice consensus guidelines 2018: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 19(Suppl 27):47–63
Beltrand J, Busiah K, Vaivre-Douret L et al (2020) Neonatal diabetes mellitus. Front Paediatr 8:540718
Busiah, K., Drunat, S., Vaivre-Douret, L., et al. French NDM study group (2013). Neuropsychological dysfunction and developmental defects associated with genetic changes in infants with neonatal diabetes mellitus: a prospective cohort study [corrected]. The lancet. Diabetes & endocrinology, 1(3), 199–207
Bowman, P., Mathews, F., Barbetti, F., et al. & Neonatal Diabetes International Collaborative Group (2021). Long-term Follow-up of Glycemic and Neurological Outcomes in an International Series of Patients With Sulfonylurea-Treated ABCC8 Permanent Neonatal Diabetes. Diabetes care, 44(1), 35–42
Cao B, Gong C, Wu D et al (2016) Genetic analysis and follow-up of 25 neonatal diabetes mellitus patients in China. J Diabetes Res 2016:6314368
Russo, L., Iafusco, D., Brescianini, S., et al. & ISPED Early Diabetes Study Group (2011). Permanent diabetes during the first year of life: multiple gene screening in 54 patients. Diabetologia, 54(7), 1693–1701
Demirbilek H, Arya VB, Ozbek MN et al (2015) Clinical characteristics and molecular genetic analysis of 22 patients with neonatal diabetes from the South-Eastern region of Turkey: predominance of non-KATP channel mutations. Eur J Endocrinol 172(6):697–705
De Franco E, Flanagan SE, Houghton JA et al (2015) The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet (London, England) 386(9997):957–963
Ellard S, Lango Allen H, De Franco E et al (2013) Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia 56(9):1958–1963
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med: Off J Am Coll Med Genet 17(5):405–424
Habeb AM, Al-Magamsi MS, Eid IM et al (2012) Incidence, genetics, and clinical phenotype of permanent neonatal diabetes mellitus in northwest Saudi Arabia. Pediatr Diabetes 13(6):499–505
Al-Khawaga S, Mohammed I, Saraswathi S et al (2019) The clinical and genetic characteristics of permanent neonatal diabetes (PNDM) in the state of Qatar. Mol Genet Genomic Med 7(10):e00753
Suzuki S, Makita Y, Mukai T, Matsuo K, Ueda O, Fujieda K (2007) Molecular basis of neonatal diabetes in Japanese patients. J Clin Endocrinol Metab 92(10):3979–3985
Al Senani A, Hamza N, Al Azkawi H et al (2018) Genetic mutations associated with neonatal diabetes mellitus in OMANI patients. J Pediatric Endocrinol Metab: JPEM 31(2):195–204
Deeb A, Habeb A, Kaplan W et al (2016) Genetic characteristics, clinical spectrum, and incidence of neonatal diabetes in the emirate of AbuDhabi, United Arab Emirates. Am J Med Genet A 170(3):602–609
Rubio-Cabezas, O., Patch, A. M., Minton, J. A., et al. Neonatal Diabetes International Collaborative Group, Hattersley, A. T., & Ellard, S. (2009). Wolcott-Rallison syndrome is the most common genetic cause of permanent neonatal diabetes in consanguineous families. The Journal of clinical endocrinology and metabolism, 94(11), 4162–4170
Garin I, Edghill EL, Akerman I et al (2010) Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci United States of America 107(7):3105–3110
Grulich-Henn J, Wagner V, Thon A et al (2010) Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV). Diabetic Med: A J Br Diabetic Assoc 27(6):709–712
Støy, J., Greeley, S. A., Paz, V. P., et al. & United States Neonatal Diabetes Working Group (2008). Diagnosis and treatment of neonatal diabetes: a United States experience. Pediatric diabetes, 9(5), 450–459
Denkboy Öngen Y, Eren E, Demirbaş Ö et al (2021) Genotype and phenotype heterogeneity in neonatal diabetes: a single centre experience in Turkey. J Clin Res Pediatric Endocrinol 13(1):80–87
Welters A, Meissner T, Konrad K et al (2020) Diabetes management in wolcott-rallison syndrome: analysis from the German/Austrian DPV database. Orphanet J Rare Dis 15(1):100
Polak, M., Dechaume, A., Cavé, H., et al. & French ND (Neonatal Diabetes) Study Group (2008). Heterozygous missense mutations in the insulin gene are linked to permanent diabetes appearing in the neonatal period or in early infancy: a report from the French ND (Neonatal Diabetes) Study Group. Diabetes, 57(4), 1115–1119
Rafiq, M., Flanagan, S. E., Patch, A. M., Shields, B. M., Ellard, S., Hattersley, A. T., & Neonatal Diabetes International Collaborative Group (2008). Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes care, 31(2), 204–209
Osbak KK, Colclough K, Saint-Martin C et al (2009) Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat 30(11):1512–1526
Njølstad PR, Søvik O, Cuesta-Muñoz A et al (2001) Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med 344(21):1588–1592
Ganie MA, Ali I, Ahangar AG et al (2012) Thiamine responsive megaloblastic anemia syndrome associated with patent ductus arteriosus: first case report from Kashmir Valley of the Indian subcontinent. Indian J Endocrinol Metab 16(4):646–650
Huggard, D., Stack, T., Satas, S., & Gorman, C. O. (2015). Donohue syndrome and use of continuous subcutaneous insulin pump therapy. BMJ case reports, 2015, bcr2015210019
Bacchetta R, Barzaghi F, Roncarolo MG (2018) From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci 1417(1):5–22
Acknowledgements
We are grateful to all the families that participated in this study. Genetic testing performed at the University of Exeter Medical School was funded through a Wellcome Trust Senior Investigator Award (098395/Z/12/Z).
Funding
Genetic testing performed at the University of Exeter Medical School was funded through a Wellcome Trust Senior Investigator Award (098395/Z/12/Z). EDF is a Diabetes UK RD Lawrence fellow. SEF has a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (105636/Z/14/Z).
Author information
Authors and Affiliations
Contributions
W.L. initiated the main study idea. W.L., M.E., A.E., A.E., N.S., H.A., and M.A. contributed to the study design. W.L. collected patients’ samples, collected, and analysed the data. S.E.F. and E.D.F. analysed the genetic data. W.L. wrote the first draft of the manuscript. M.E., A.E., A.E., N.S., H.A., S.E.F., E.D.F., and M.A. revised and edited the manuscript. All the authors approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This report has been approved by institutional review board and was in accordance with the 1964 Helsinki declaration and its later amendments.
Consent to participate
Informed written consents to publish the cases have been obtained from the patients’ parents.
Consent for publication
Patients signed informed consent regarding publishing their data. Parents of patient 14.1 signed informed consent for publication of Supplementary Fig. 1 and Supplementary Fig. 2.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
It shows acanthosis nigricans at neck, axilla and groin, prominent nipple, hypertrichosis and clitromegaly
Supplementary file2 (TIF 31212 KB)
592_2021_1788_MOESM3_ESM.tif
A: It shows prominent infraorbital creases, low set ears, thick lips, and acanthosis nigricans. B: It shows umbilical hernia and abdominal distension
Supplementary file3 (TIF 17586 KB)
Rights and permissions
About this article
Cite this article
Laimon, W., El-Ziny, M., El-Hawary, A. et al. Genetic and clinical heterogeneity of permanent neonatal diabetes mellitus: a single tertiary centre experience. Acta Diabetol 58, 1689–1700 (2021). https://doi.org/10.1007/s00592-021-01788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01788-6