Abstract
Aim
Women with prior gestational diabetes (GDM) are at increased diabetes risk. This study aimed to assess whether lifestyle is associated with glycemic health of high-risk women 5 years postpartum, taking into account the pre-pregnancy BMI.
Methods
The RADIEL study enrolled before or in early pregnancy 720 women with pre-pregnancy BMI ≥ 30 kg/m2 and/or prior GDM. The follow-up visit 5 years postpartum included questionnaires and measurements of anthropometrics, blood pressure, and physical activity (PA) as well as analyses of glucose metabolism, lipids, and inflammatory markers. We measured body composition (Inbody) and calculated a Healthy Food Intake Index (HFII) from Food Frequency Questionnaires (FFQ). ArmBand measured PA, sedentary time, and sleep. To take into account the diverse risk groups of GDM, we divided the women based on pre-pregnancy BMI over/under 30 kg/m2.
Results
Altogether 348 women attended the follow-up. The obese and non-obese women showed similar prevalence of glycemic abnormalities, 13% and 19% (p = 0.139). PA levels were higher among the non-obese women (p < 0.05), except for step count, and their HFII was higher compared to the obese women (p = 0.033). After adjusting for age, education, and GDM history, PA and HFII were associated with glycemic health only among obese women. When both lifestyle factors were in the same model, only PA remained significant. PA associated with other markers of metabolic health also among the non-obese women, excluding HbA1c.
Conclusion
Lifestyle 5 years postpartum was associated with better glycemic health only among the obese high-risk women. PA, however, is essential for the metabolic health of all high-risk women.
Clinical trial registration
ClinicalTrials.gov, http://www.clinicaltrials.com, NCT01698385.
Similar content being viewed by others
Introduction
The escalating epidemic of obesity and diabetes [1] requires efficient methods for prevention. Fortunately, according to large international studies, type 2 diabetes (T2D) is preventable among high-risk groups with lifestyle intervention [2, 3]. Women with a history of gestational diabetes (GDM) are at a sevenfold risk of developing future diabetes [4, 5] and therefore many intervention studies have recently focused also on women with prior GDM [6]. For example, the RADIEL (The Finnish Gestational Diabetes Prevention) lifestyle intervention starting during pregnancy and continuing during the 1st postpartum year successfully reduced the incidence of glycemic abnormalities 1 year after delivery [7].
T2D and GDM, however, are heterogeneous diseases. Scandinavian researchers recently categorized T2D into 5 subclasses [8], each with varying characteristics, complications, and progression. The heterogeneity of GDM has received less interest, but in addition to T2D, also women with GDM differ by their body composition, insulin secretion and resistance, as well as diabetes-related autoimmunity [9,10,11,12]. In the RADIEL study, the incidence of glycemic abnormalities was high 5 years postpartum also among the non-obese women with prior GDM, and they presented often with normal-weight obesity (NWO), i.e., normal body mass index (BMI) but high body fat percentage [13].
There are suggestions that physical inactivity, smoking, alcohol consumption, and poorer dietary habits of NWO individuals could explain their impaired metabolic health [14]. This could offer one possible explanation for the high prevalence of glycemic abnormalities postpartum also among the non-obese GDM women. On the other hand, non-obese women with GDM have typically a deficiency in insulin production [15] and we hypothesize that lifestyle changes aiming at reducing insulin resistance by increasing physical activity (PA) and improving diet quality might not have similar effect among non-obese and obese women. The aim of this study was to assess the lifestyle of women at high risk for diabetes 5 years postpartum and to evaluate the associations between lifestyle and glycemic health in GDM subgroups, i.e., obese and non-obese women based on their pre-pregnancy BMI over/under 30 kg/m2.
Materials and methods
Study design
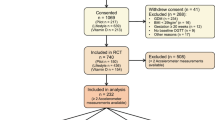
This is a cross-sectional study focusing on the participants of the RADIEL 5-year follow-up study. Originally, the RADIEL lifestyle intervention study was a randomized controlled trial (RCT), recruiting women at high risk for GDM before or in early pregnancy. Altogether 724 women entered the study between years 2008–2014. During years 2013–2017, 5 years after delivery, all participants with a live birth and their children were invited to a follow-up study. In the original study, some participants visited the study nurse for data collection every 3 months before pregnancy and all participants once in each trimester of pregnancy and 6 weeks, 6 and 12 months postpartum. The study visits took place in Helsinki, in the Helsinki University Hospital (HUH), and in Lappeenranta, in South Karelian Central Hospital (SKCH). Previous publications provide the details of these studies [13, 16].
The study participants were at least 18 years of age and had a BMI 30 kg/m2 or over, and/or prior GDM. Overt diabetes, medications altering glucose metabolism, multiple pregnancy, severe psychiatric problems, physical disabilities, and communication problems based on inadequate language skills led to exclusion. All participants gave a written informed consent and the ethics committees of the HUH and SKCH both approved the study protocol.
Outcomes
The main outcome of this study is the presence of glycemic abnormalities 5 years after delivery. All participants, except for those with physician-diagnosed T2D or prior bariatric surgery, underwent a 75 g 2-h oral glucose tolerance test (OGTT). T2D diagnosis was based on fasting plasma glucose ≥ 7.0 mmol/l or 2-h glucose ≥ 11.1 mmol/l. The definition for impaired glucose tolerance (IGT) was 2-h glucose 7.8–11.0 mmol/l and diagnosis of impaired fasting glucose (IFG) required fasting plasma glucose in the range of 6.1–6.9 mmol/l. Completing the criteria of either IFG, IGT, or T2D resulted in a composite outcome of glycemic abnormality.
Each study visit included measurements of body weight, height, and waist and hip circumference. The calculation for postpartum weight change derived from the difference between weight at the study visits 6 weeks and 5 years postpartum. Blood pressure was measured with a sphygmomanometer in a sitting position from the right arm. The laboratory tests in conjunction with the study visit, after a 10–12 h fast, included analysis of glucose metabolism (2 h 75 g OGTT, GHbA1c, insulin), lipids (cholesterol, low-density lipoprotein cholesterol LDL, high-density lipoprotein cholesterol HDL, and triglycerides), inflammatory markers (highly sensitive C-reactive protein hs-CRP), and alanine amino transferase (ALAT). A multi-frequency bio-impedance measurement method (InBody3.0, Biospace Co., Ltd, Seoul, Korea) provided the information on body composition [17]. AGE Reader measured the advanced-glycation end-products (AGEs), which are potential early markers of diabetes risk.
Lifestyle in this study refers to PA and diet. SenseWear ArmBand Pro 3 recorded PA, sedentary time, and sleep of the participants, who wore the monitor in their upper non-dominant arm. The monitor required removal while showering, sauna, and water-sports. To be included in the analysis, we required recording of at least 85% of a day, during a minimum of 4 days, and including at least one weekend day. We extracted measures for light PA (LPA) (min/day), moderate-to-vigorous PA (MVPA) (min/day), vigorous PA (VPA) (min/day), sleep (h/day), steps (counts/day), and sedentary time (min/day, excluding sleep).
Food frequency questionnaires (FFQ) provided the information on current diet. In the analysis, we used the daily intake of specified food groups as well as a healthy food intake index (HFII) describing the diet as an entity; participants received points reflecting their intake of high-energy/low-nutrient snacks (0–2 points), sugar-sweetened beverages (0–1 points), fast food (0–1 points), high-fiber grains (0–2 points), bread fat spread (0–2 points), low-fat cheese (0–1 points), low-fat milk (0–2 points), fish (0–2 points), red and processed meat (0–2 points), vegetables (0–2 points), and fruits and berries (0–1 points). The maximum score available was 18, with a higher score indicating a healthier diet. In case of a missing answer, the HFII score was not calculated. We used HFII both as continuous and as categorical, divided into three categories. The first cut-off was determined by subtracting 1SD from the mean and the second by adding 1SD to the mean.
Background questionnaires covered additional aspects of lifestyle, such as smoking, with regular and occasional smoking combined. Questionnaires also recorded self-reported duration of breastfeeding, years of education, family income as well as chronic illnesses. The Center for Epidemiological Studies Depression Scale (CES-D) [18] provided the tool for assessing depressive symptoms. In the analysis we used both the logarithmic sum as a continuous variable and the generally acknowledged cut-off of 16 points to indicate depression [19].
For assessing the associations between lifestyle and glycemic health, we divided the women based on their pre-pregnancy BMI over or under 30 kg/m2. The justification for using pre-pregnancy instead of current BMI was the high metabolic risk of the normal-weight women with GDM [20]. The postpartum weight change in either direction could have mixed these diverse risk groups which potentially have different pathophysiological backgrounds. When referring to subgroups based on BMI (non-obese and obese) in this article, we always refer to the pre-pregnancy BMI.
Statistics
We examined normal distribution of the variables with the Shapiro–Wilk test. Descriptive characteristics are presented as means with standard deviations (SD), medians with interquartile range (IQR) or as frequencies with percentages. Between-groups comparisons were performed with the Chi square test, the Mann–Whitney U test, ANOVA, or the independent sample T test, when appropriate. For examining the associations between glycemic abnormalities and lifestyle (MVPA and HFII) we used the binary logistic regression model. We assessed the correlations between PA and metabolic characteristics with Spearman’s rank-order correlation. We performed the statistical analyses with the SPSS 24.0 software program (SPSS Inc., Chicago, IL, USA) and considered a p value < 0.05 as statistically significant.
Results
Data on glucose metabolism were available for 348 women, HFII for 298 women, and device-measured PA for 206 women. In total 51 women (13.6%) had a glycemic abnormality; 24 women (6.4%) had IFG, 24 women (6.4%) IGT, and 13 women (3.5%) T2D.
At the follow-up visit, previously obese and non-obese women differed significantly in most measured parameters (Table 1). In addition to better metabolic health, non-obese women also showed lower depression scores. There were no differences in age, smoking, family history of diabetes, or GHbA1c between the BMI-groups. Among the obese women, 7 (3.1%) were diagnosed with T2D, 16 (7.2%) had IGT, and 13 women (5.8%) IFG. The corresponding numbers for non-obese women were 6 (4.8%), 8 (6.4%) and 11 (8.8%), respectively. There was no statistically significant difference in the prevalence of IFG, IGT, or T2D between the groups. The indices for insulin resistance (HOMA-IR) and insulin secretion (HOMA-β) were significantly different: previously obese women showed higher HOMA-IR and HOMA-β levels.
Table 2 presents the lifestyle measurements 5 years after delivery. The non-obese women slept more, had less sedentary time, and higher PA levels, except for the step count, which did not differ between the groups. They also reported to consume less often snacks (p = 0.043) and more often high-fiber grains (p = 0.011), and also their HFII was statistically significantly higher indicating a healthier diet compared to the obese women (p = 0.033).
In the total study population, there was a detectable association between glycemic abnormalities and age [crude OR 1.120 (1.044, 1.201) p = 0.002], years of education [crude OR 0.667 (0.449, 0.990) p = 0.044], body fat percentage [crude OR 1.044 (1.066, 1.085) p = 0.025], postpartum weight change [crude OR 1.055 (1.020, 1.090) p = 0.002], GDM history [crude OR 5.181 (1.998, 13.435) p = 0.001] and current BMI [crude OR 1.058 (1.012, 1.107) p = 0.012]. There was no association between glycemic abnormalities and RADIEL lifestyle intervention, depression, breastfeeding, diet, physical activity, sleep, or family income. Based on these results, we adjusted the further logistic regression models with GDM history, age, and education years. Body fat percentage and postpartum weight change were not included, as they can be considered as mediators of the lifestyle effects.
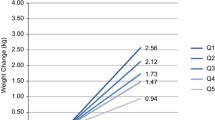
There was an interaction between pre-pregnancy BMI and MVPA (p = 0.009) on glycemic abnormalities (Fig. 1), but not between pre-pregnancy BMI and diet. Table 3 presents the multivariable regression models among obese and non-obese women. Lifestyle was associated with glycemic health only among women with pre-pregnancy BMI 30 kg/m2 or higher; after adjusting with age, education, and GDM history, both MVPA and HFII were associated with glycemic health. When adjusted additionally with current BMI and analyzing both lifestyle factors in the same model, only PA remained significant. When performing the regression analysis with HFII as a categorical variable the results were similar (data not shown).
Moderate-to-vigorous physical activity (MVPA min/day) in groups of previously non-obese and obese women, according to presence of glycemic abnormalities. Interaction p = 0.009. For the clarity of the figure, two extremely high outliers were excluded from the group of non-obese women without glycemic abnormality (383 min/day and 360 min/day)
To further assess the importance of PA in phenotypically diverse groups, we evaluated the correlations between measures of PA, sedentary time, and sleep and markers of metabolic health. Table 4 presents these correlations, adjusted with age, education years, and GDM history, separately among obese and non-obese women. Among obese women, VPA, MVPA, LPA, and sedentary time correlated with all markers of metabolic health. Step count correlated with body fat percentage, HDL, insulin, and blood pressure. Among non-obese women VPA and MVPA correlated with all other markers of metabolic health, except for GHbA1c.
Discussion
The association between lifestyle and glycemic health varies between obese and non-obese high-risk women. In our study, the non-obese women were physically more active and had a healthier diet compared to obese women. Despite their healthier lifestyle, however, they presented with similar prevalence of glycemic abnormalities 5 years postpartum. When adjusted for age, education, and GDM history, PA and diet were associated with better glycemic health only among the obese women. This remained significant even when adjusted with current BMI. However, higher levels of MVPA and VPA were associated with numerous other components of metabolic health, such as lower body fat percentage, blood pressure, and healthier lipid profile, also among the non-obese high-risk women.
Although previous studies have suggested that NWO individuals might have poorer dietary habits, this was not true in our population. The non-obese women had overall a higher HFII indicating a healthier diet compared to obese participants. They also consumed less snacks and more whole grain products, which have been suspected to improve the function of beta cells [21], and there was a trend toward lower consumption of red meats, generally associated with lower diabetes risk [22]. The HFII of non-obese women, however, was not associated with glycemic health 5 years postpartum.
The non-obese participants were also physically more active. PA is generally associated with lower morbidity and mortality [23] and device-measured PA has shown even a stronger association with health benefits [24]. The influence of PA may be at least partly mediated through reductions in total and abdominal fat leading to improvements in insulin sensitivity and blood pressure, and possibly improvements in endothelial vascular function [25]. Our results are in line with previous studies as PA was associated with blood pressure, body fat percentage, lipids, waist circumference, insulin, and hs-CRP among all participants.
In our study there was, however, a significant interaction between PA and pre-pregnancy BMI when assessing glycemic abnormalities. Also a few other studies have assessed the influence of lifestyle in phenotypically different subgroups. An Indian study demonstrated that weight loss is beneficial also among non-obese individuals with family history of diabetes [26]. In preventing the metabolic syndrome (MetS), PA seems similarly effective among non-obese individuals [27], which has been demonstrated also elsewhere [28].
Nevertheless, there are studies reporting similar interactions in general population as in our study. In two large cohort studies, PA was associated with less visceral adipose tissue but this association attenuated after adjustment for BMI (32) and also the association between PA and BMI appeared weaker among non-obese individuals [29]. A study on 1109 adults, using accelerometer-measured PA, also demonstrated an interaction between BMI and association of MVPA and HbA1c [30].
On the other hand, the correlations between PA and metabolic parameters were similar among the obese and non-obese. The strongest correlation was with body fat percentage, waist circumference, and blood pressure. This is reassuring as lifestyle may influence the important cardiovascular risk factors also among the non-obese, which has been reported also previously [31]. In our study, among non-obese women, however, associations existed only with more strenuous PA whereas even lower intensity PA was associated with better metabolic outcome among the obese. These results additionally supported our findings concerning glycemic abnormalities, as HbA1c and PA were not correlated among non-obese women.
A few reasons have been suggested to explain the heterogeneous response to lifestyle. Gene-lifestyle interactions may influence the susceptibility to weight gain and effectiveness of lifestyle interventions [32, 33]. These interactions have been demonstrated in large studies, e.g., polymorphisms in the PPAR-gamma2 gene and Glu9 [33], as well as in the MTNR1B, which also decreased the effect of the RADIEL intervention in preventing GDM [34]. Polymorphisms in the PPAR-gamma2 gene, e.g., modify the effects of lifestyle on weight loss, lipids, and insulin sensitivity [33]. In the Finnish Diabetes Prevention Study certain polymorphisms in GHRL and LEPR genes modified the effect of PA on weight, waist circumference, lipids, and blood pressure, but had no effect on the progression from IGT to T2D [35]. Additionally, polymorphisms in the TNF-α gene may interfere with the anti-inflammatory effect of PA [36].
In addition to genetic polymorphisms, also other background factors might interfere with the effects of lifestyle. It is estimated that 5–6% of GDM women have some form of monogenetic diabetes (MODY) [37, 38], which will not respond to lifestyle changes similarly. Lifestyle changes might also have a stronger effect among people with insulin resistance [39]. Hypothetically, the adiposity-related insulin resistance might be more reversible than the defects in insulin secretion. In our study, the previously non-obese women showed significantly lower insulin secretion indices, generally associated with higher T2D risk [40]. Additionally, gut microbiota is known to influence the risk of developing diabetes in an interplay with diet [41].
Strengths of this study are the inclusion of phenotypically different groups of high-risk women. We also have detailed device-measured data on PA as well as detailed dietary information from FFQs and diet diaries. Additionally, having measured multiple metabolic markers, including body composition, enables us to analyze not only glycemic disorders but also other aspects of metabolic health such as body fat percentage. Limitations of our study include the number of participants, especially when divided into subgroups, and lack of a control group to enable comparison with normal unselected population. Due to our study design, all non-obese participants had a history of GDM indicating a higher risk of glycemic abnormalities also postpartum. This might potentially influence our findings. Also, all participants were of Caucasian origin and this limits the generalizability of our findings.
Although intervention studies in big populations have reached well-documented positive results [6, 42], it is important to assess components of lifestyle individually and in phenotypically distinct groups to further develop our recommendations for high-risk individuals. Our study demonstrated that healthy lifestyle is essential for high-risk women, but among non-obese women it was not associated with better glycemic health. PA, however, has a positive association with many other markers of metabolic health also among the non-obese, and is therefore most probably beneficial for their cardiovascular health.
We showed that higher levels of PA are associated with better metabolic health. However, other factors than lifestyle may play a role for the glycemic health of non-obese women. It is important to recognize this high-risk group of non-obese women with prior GDM, as even their physically active lifestyle and healthier diet is not a marker of lower diabetes risk. Equally important is to study the factors behind their increased risk and to find new innovative ways to prevent T2D. There is a need for precision medicine approach in diabetes research and treatment [8, 43] and studies focusing on genetic polymorphisms, microbiome, and metabolomics might provide new insights in the future. On the other hand, even more emphasis should be put on motivational lifestyle interventions among the obese women as T2D could be prevented. Perhaps individualized counseling, social support, self-efficacy, and listening to women’s wishes [44] require further attention to improve the results of lifestyle interventions [45]. As T2D is an expanding epidemic, we cannot afford to neglect this group of high-risk women.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Risk NC, Factor collaboration (NCD-RisC) (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 44 million participants. Lancet 387(10027):1513–1530. https://doi.org/10.1016/s0140-6736(16)00618-8
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403
Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344(18):1343–1350
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
Kramer CK, Campbell S, Retnakaran R (2019) Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 62(6):905–914. https://doi.org/10.1007/s00125-019-4840-2
Goveia P, Canon-Montanez W, Santos DP et al (2018) Lifestyle intervention for the prevention of diabetes in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 9:583. https://doi.org/10.3389/fendo.2018.00583
Huvinen E, Koivusalo SB, Meinila J, Valkama A, Tiitinen A, Rono K, et al. Effects of a Lifestyle Intervention During Pregnancy and First Postpartum Year: Findings From the RADIEL Study. Journal of Clinical Endocrinology & Metabolism. 2018;103(4):1669-77
Ahlqvist E, Storm P, Karajamaki A et al (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(18)30051-2
Freinkel N, Metzger BE, Phelps RL et al (1985) Gestational diabetes mellitus. Heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes 34(Suppl 2):1–7
Powe CE, Allard C, Battista MC et al (2016) Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39(6):1052–1055. https://doi.org/10.2337/dc15-2672
Huvinen E, Grotenfelt NE, Eriksson JG et al (2016) Heterogeneity of maternal characteristics and impact on gestational diabetes (GDM) risk-Implications for universal GDM screening? Ann Med 48(1–2):52–58. https://doi.org/10.3109/07853890.2015.1131328
Huvinen E, Eriksson JG, Stach-Lempinen B, Tiitinen A, Koivusalo SB (2018) Heterogeneity of gestational diabetes (GDM) and challenges in developing a GDM risk score. Acta Diabetol 55(12):1251–1259. https://doi.org/10.1007/s00592-018-1224-x
Huvinen E, Eriksson JG, Koivusalo SB et al (2018) Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: findings from the RADIEL study follow-up. Acta Diabetol 55(5):493–501. https://doi.org/10.1007/s00592-018-1118-y
Mannisto S, Harald K, Kontto J et al (2014) Dietary and lifestyle characteristics associated with normal-weight obesity: the National FINRISK 2007 Study. Br J Nutr 111(5):887–894. https://doi.org/10.1017/S0007114513002742
Damm P, Kuhl C, Hornnes P, Molsted-Pedersen L (1995) A longitudinal study of plasma insulin and glucagon in women with previous gestational diabetes. Diabetes Care 18(5):654–665
Rono K, Stach-Lempinen B, Klemetti MM et al (2014) Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 14:70. https://doi.org/10.1186/1471-2393-14-70
Malavolti M, Mussi C, Poli M et al (2003) Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol 30(4):380–391. https://doi.org/10.1080/0301446031000095211
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401. https://doi.org/10.1177/014662167700100306
Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ (1977) Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 106(3):203–214. https://doi.org/10.1093/oxfordjournals.aje.a112455
Huvinen E, Eriksson JG, Koivusalo SB et al (2018) Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: findings from the RADIEL study follow-up. Acta Diabetol 19:19. https://doi.org/10.1007/s00592-018-1118-y
Malin SK, Kullman EL, Scelsi AR, Godin JP, Ross AB, Kirwan JP (2019) A whole-grain diet increases glucose-stimulated insulin secretion independent of gut hormones in adults at risk for type 2 diabetes. Mol Nutr Food Res 63(7):e1800967. https://doi.org/10.1002/mnfr.201800967
Pan A, Sun Q, Bernstein AM et al (2011) Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 94(4):1088–1096. https://doi.org/10.3945/ajcn.111.018978
Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr (1991) Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J 1(3):147–152
Lee IM, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE (2018) Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: the women’s health study. Circulation 137(2):203–205. https://doi.org/10.1161/CIRCULATIONAHA.117.031300
Stewart KJ (2002) Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA 288(13):1622–1631
Viswanathan M, Snehalatha C, Viswanathan V, Vidyavathi P, Indu J, Ramachandran A (1997) Reduction in body weight helps to delay the onset of diabetes even in non-obese with strong family history of the disease. Diabetes Res Clin Pract 35(2–3):107–112
de Rooij BH, van der Berg JD, van der Kallen CJ et al (2016) Physical activity and sedentary behavior in metabolically healthy versus unhealthy obese and non-obese individuals—the maastricht study. PLoS One [Electron Resour] 11(5):e0154358. https://doi.org/10.1371/journal.pone.0154358
Zhu S, St-Onge MP, Heshka S, Heymsfield SB (2004) Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metab Clin Exp 53(11):1503–1511
Hemmingsson E, Ekelund U (2007) Is the association between physical activity and body mass index obesity dependent? Int J Obes 31(4):663–668
Bakrania K, Yates T, Edwardson CL et al (2017) Associations of moderate-to-vigorous-intensity physical activity and body mass index with glycated haemoglobin within the general population: a cross-sectional analysis of the 2008 Health Survey for England. BMJ Open 7(4):e014456. https://doi.org/10.1136/bmjopen-2016-014456
Lefevre M, Redman LM, Heilbronn LK et al (2009) Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis 203(1):206–213. https://doi.org/10.1016/j.atherosclerosis.2008.05.036
Goni L, Cuervo M, Milagro FI, Martinez JA (2014) Gene-gene interplay and gene-diet interactions involving the MTNR1B rs10830963 variant with body weight loss. J Nutrigenet Nutrigenom 7(4–6):232–242. https://doi.org/10.1159/000380951
Uusitupa M (2005) Gene-diet interaction in relation to the prevention of obesity and type 2 diabetes: evidence from the Finnish Diabetes Prevention Study. Nutr Metab Cardiovasc Dis 15(3):225–233
Grotenfelt NE, Wasenius NS, Rono K et al (2016) Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia 59(8):1655–1658. https://doi.org/10.1007/s00125-016-3989-1
Kilpelainen TO, Lakka TA, Laaksonen DE et al (2008) Interaction of single nucleotide polymorphisms in ADRB2, ADRB3, TNF, IL6, IGF1R, LIPC, LEPR, and GHRL with physical activity on the risk of type 2 diabetes mellitus and changes in characteristics of the metabolic syndrome: the finnish diabetes prevention study. Metab Clin Exp 57(3):428–436. https://doi.org/10.1016/j.metabol.2007.10.022
Kilpelainen TO, Laaksonen DE, Lakka TA et al (2010) The rs1800629 polymorphism in the TNF gene interacts with physical activity on the changes in C-reactive protein levels in the Finnish Diabetes Prevention Study. Exp Clin Endocrinol Diabetes 118(10):757–759. https://doi.org/10.1055/s-0030-1249686
Ellard S, Beards F, Allen LI et al (2000) A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia 43(2):250–253. https://doi.org/10.1007/s001250050038
Dickens LT, Letourneau LR, Sanyoura M, Greeley SAW, Philipson LH, Naylor RN (2019) Management and pregnancy outcomes of women with GCK-MODY enrolled in the US Monogenic Diabetes Registry. Acta Diabetol 56(4):405–411. https://doi.org/10.1007/s00592-018-1267-z
Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J (2003) Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance. 4-Year results from the finnish diabetes prevention study. Diabetes 52(10):2532–2538. https://doi.org/10.2337/diabetes.52.10.2532
Song Y, Manson JE, Tinker L et al (2007) Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care 30(7):1747–1752. https://doi.org/10.2337/dc07-0358
Tuomainen M, Lindstrom J, Lehtonen M et al (2018) Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes 8(1):35. https://doi.org/10.1038/s41387-018-0046-9
Laaksonen DE, Lindstrom J, Lakka TA et al (2005) Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 54(1):158–165
Wang DD, Hu FB (2018) Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol 6(5):416–426. https://doi.org/10.1016/S2213-8587(18)30037-8
Eades CE, France EF, Evans JMM (2018) Postnatal experiences, knowledge and perceptions of women with gestational diabetes. Diabet Med 35(4):519–529. https://doi.org/10.1111/dme.13580
Koh D, Miller YD, Marshall AL, Brown WJ, McIntyre D (2010) Health-enhancing physical activity behaviour and related factors in postpartum women with recent gestational diabetes mellitus. J Sci Med Sport 13(1):42–45. https://doi.org/10.1016/j.jsams.2008.10.003
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital.
Funding
The study was funded by The Finnish Medical Foundation, Alfred Kordelin Foundation, Juho Vainio Foundation, Ahokas Foundation, the Finnish Foundation for Cardiovascular Disease, Academy of Finland, Special state subsidy for health science research of Helsinki University Hospital (HUH), Samfundet Folkhälsan, Finska Läkaresällskapet, Viipuri Tuberculosis Foundation, The Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, Health Promotion Grant (Ministry of Social Affairs and Health) EU H2020-PHC-2014-DynaHealth Grant no. 633595 and The Social Insurance Institution of Finland. The funders have not had any role in designing or conducting the study; nor in collection, management, analysis, or interpretation of the data; nor in preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
EH participated in the design and implementation of the study, literature search, data interpretation, and the drafting and editing of the article. SBK the principal investigator of the study, initiated, participated in the design of, and coordinated the study, and participated in the analysis of the results, and advised on drafting and editing the article. EE participated in the design of the study, in the analysis of the results, and in drafting and editing of the article. JM participated in the design of the study, helped in the analysis of the dietary data, and participated in editing the article. TT and JK participated in the design of the study, were responsible for the analysis of the physical activity data, and helped with the editing of the article. KH participated in the design of the study, helped in the analysis of psychological data, and participated in editing the article. PB participated in the design of the study, statistical analysis of the data, and editing the article. BS-L participated in the design of the study, coordinated the study in Lappeenranta, and helped with editing the article. All authors have read and approved the final version of the manuscript. EH is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this manuscript.
Ethical approval
The Ethics Committees of Helsinki University Central Hospital (Dnro 300/E9/06) and South-Karelia Central Hospital (Dnro M06/08) both approved the study protocol.
Informed consent
All participants gave a written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Pregnancy and Diabetes, managed by Antonio Secchi and Marina Scavini.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huvinen, E., Engberg, E., Meinilä, J. et al. Lifestyle and glycemic health 5 years postpartum in obese and non-obese high diabetes risk women. Acta Diabetol 57, 1453–1462 (2020). https://doi.org/10.1007/s00592-020-01553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01553-1