Abstract
Aims
5-Aminoimidazole-4-carboxamide riboside (AICAR) is an endogenous activator of AMPK, a central regulator of energy homeostasis. Loss and/or reduction of AMPK signaling plays an important role in the development of insulin resistance in type 2 diabetes. The loss of AMPK in diabetes could be due to a loss of AICAR. The aim of this study was to characterize urine levels of AICAR in diabetes and determine whether an association exists with respect to late complications, e.g., retinopathy, nephropathy and neuropathy.
Methods
Urine AICAR was measured by liquid chromatography tandem mass spectrometry in 223 patients consisting of 5 healthy controls, 63 patients with pre-diabetes, 29 patients with newly diagnosed type 2 diabetes and 126 patients with long-standing type 2 diabetes. For statistical analyses, nonparametric Kruskal–Wallis test, one-way ANOVA and multivariate regression analysis were performed to investigate the associations of urinary AICAR excretion within different groups and different clinical parameters.
Results
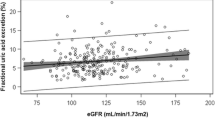
The mean urine AICAR for all 223 patients was 694.7 ± 641.1 ng/ml. There was no significant difference in urine AICAR between the control and patients with diabetes (592.3 ± 345.1 vs. 697.1 ± 646.5 ng/ml). No association between any of the biochemical and/or clinical parameters measured and urine AICAR was found, with the exception of age of patient (R = − 0.34; p < 0.01) and estimated glomerular filtration rate (R = 0.19; p = 0.039). These results were confirmed additionally by linear regression analysis.
Conclusions
Clinical diabetes is not associated with a change in endogenous AICAR levels. Loss of AICAR may therefore not be a mechanism by which AMPK signaling is reduced in diabetes.


Similar content being viewed by others
Abbreviations
- ACR:
-
Albumin to creatinine ratio
- AICAR:
-
5-Aminoimidazole-4-carboxamide riboside
- AMPK:
-
AMP-activated protein kinase
- ASA:
-
Acetylsalicylic acid
- BMI:
-
Body mass index
- CBS:
-
Cystathionine beta-synthase
- CVD:
-
Cardiovascular disease
- HCT:
-
Hydrochlorothiazide
- NSS:
-
Neuropathy symptom score (NSS)
- NDS:
-
Neuropathy disability score (NDS)
- RAAS:
-
Renin–angiotensin–aldosterone system
References
Ruderman N, Prentki M (2004) AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3(4):340–351. https://doi.org/10.1038/nrd1344
Ruderman NB, Carling D, Prentki M, Cacicedo JM (2013) AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123(7):2764–2772. https://doi.org/10.1172/JCI67227
Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM (2006) Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55(8):2277–2285. https://doi.org/10.2337/db06-0062
Xu XJ, Gauthier MS, Hess DT et al (2012) Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res 53(4):792–801. https://doi.org/10.1194/jlr.P022905
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229(2):558–565
Sullivan JE, Carey F, Carling D, Beri RK (1994) Characterisation of 5′-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5′-AMP analogues on enzyme activity. Biochem Biophys Res Commun 200(3):1551–1556
Davies SP, Helps NR, Cohen PT, Hardie DG (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 377(3):421–425. https://doi.org/10.1016/0014-5793(95)01368-7
Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG (1995) 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem 270(45):27186–27191
Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D (2006) Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281(43):32207–32216. https://doi.org/10.1074/jbc.M606357200
Bergeron R, Previs SF, Cline GW et al (2001) Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 50(5):1076–1082
Buhl ES, Jessen N, Schmitz O et al (2001) Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50(1):12–17
Iglesias MA, Ye JM, Frangioudakis G et al (2002) AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes 51(10):2886–2894
Song XM, Fiedler M, Galuska D et al (2002) 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia 45(1):56–65. https://doi.org/10.1007/s001250200006
Barre L, Richardson C, Hirshman MF et al (2007) Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. Am J Physiol Endocrinol Metab 292(3):E802–E811. https://doi.org/10.1152/ajpendo.00369.2006
Goodyear LJ (2008) The exercise pill–too good to be true? N Engl J Med 359(17):1842–1844. https://doi.org/10.1056/NEJMcibr0806723
Narkar VA, Downes M, Yu RT et al (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134(3):405–415. https://doi.org/10.1016/j.cell.2008.06.051
World Anti-Doping Agency (2009) The 2009 prohibited list international standard https://www.wada-ama.org/sites/default/files/resources/files/WADA_Prohibited_List_2009_EN.pdf. Accessed 02 Feb 2018
Thomas A, Beuck S, Eickhoff JC et al (2010) Quantification of urinary AICAR concentrations as a matter of doping controls. Anal Bioanal Chem 396(8):2899–2908. https://doi.org/10.1007/s00216-010-3560-8
Middleton JE, Coward RF, Smith P (1964) Urinary excretion of A.I.C. in vitamin-B12 and folic-acid deficiencies. Lancet 284(7353):258–259. https://doi.org/10.1016/S0140-6736(64)90216-8
Lulenski G, Donaldson M, Newcombe D (1970) Urinary aminoimidazolecarboxamide levels in children with acute leukemia. Pediatrics 45(6):983–995
Newcombe DS (1970) The urinary excretion of aminoimidazolecarboxamide in the Lesch–Nyhan syndrome. Pediatrics 46(4):508–512
Sweetman L, Nyhan WL (1970) Detailed comparison of the urinary excretion of purines in a patient with the Lesch–Nyhan syndrome and a control subject. Biochem Med 4(2):121–134
Lamia KA, Sachdeva UM, DiTacchio L et al (2009) AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326(5951):437–440. https://doi.org/10.1126/science.1172156
Pokrywka A, Cholbinski P, Kaliszewski P, Kowalczyk K, Konczak D, Zembron-Lacny A (2014) Metabolic modulators of the exercise response: doping control analysis of an agonist of the peroxisome proliferator-activated receptor delta (GW501516) and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). J Physiol Pharmacol 65(4):469–476
Hartmann S, Okun JG, Schmidt C et al (2006) Comprehensive detection of disorders of purine and pyrimidine metabolism by HPLC with electrospray ionization tandem mass spectrometry. Clin Chem 52(6):1127–1137. https://doi.org/10.1373/clinchem.2005.058842
Thomas A, Vogel M, Piper T et al (2013) Quantification of AICAR-ribotide concentrations in red blood cells by means of LC-MS/MS. Anal Bioanal Chem 405(30):9703–9709. https://doi.org/10.1007/s00216-013-7162-0
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Dyck PJ (1988) Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve 11(1):21–32. https://doi.org/10.1002/mus.880110106
Dyck PJ, Sherman WR, Hallcher LM et al (1980) Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol 8(6):590–596. https://doi.org/10.1002/ana.410080608
Quattrini C, Tavakoli M, Jeziorska M et al (2007) Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56(8):2148–2154. https://doi.org/10.2337/db07-0285
Henry RJ (1964) Non-protein nitrogenous constituents. In: Henry RJ (ed) Clinical chemistry, principles and techniques. Harper & Row, New York, pp 292–299
Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI (2017) Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig 8(1):6–18. https://doi.org/10.1111/jdi.12533
Allen SV, Hopkins WG (2015) Age of peak competitive performance of elite athletes: a Systematic review. Sports Med 45(10):1431–1441. https://doi.org/10.1007/s40279-015-0354-3
Fogarty S, Hardie DG (1804) Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta 3:581–591. https://doi.org/10.1016/j.bbapap.2009.09.012
Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G (1991) Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40(10):1259–1266
Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF (2003) 5-Aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol 284(4):R936–R944. https://doi.org/10.1152/ajpregu.00319.2002
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253. https://doi.org/10.2147/DMSO.S43731
Acknowledgements
The authors would like thank Kathrin Schmidt for her excellent technical assistance.
Authors’ contributions
MM and PPN designed the study, interpreted data and drafted the manuscript. JBG contributed substantially in the acquisition of data and revised the manuscript critically for important intellectual content. SK contributed substantially in the analysis and interpretation of data and preparing the tables and figures. CR revised the manuscript critically for important intellectual content. THF contributed substantially to the interpretation of data and revised the manuscript elaborately. JGO established the AICAR measurement, interpreted the data and revised the manuscript for important intellectual content. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was supported by the DFG (CRC1118 to MM, CR, THF & PPN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
Urine samples were obtained from three studies that have been approved by the Ethics Committee Heidelberg (S-245/2013, S-232-2013, S-383/2016) that have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All patients gave their written consent prior to their inclusion in the studies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mendler, M., Kopf, S., Groener, J.B. et al. Urine levels of 5-aminoimidazole-4-carboxamide riboside (AICAR) in patients with type 2 diabetes. Acta Diabetol 55, 585–592 (2018). https://doi.org/10.1007/s00592-018-1130-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1130-2