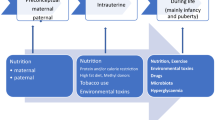
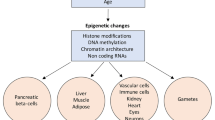
Abstract
The known genetic variability (common DNA polymorphisms) does not account either for the current epidemics of type 2 diabetes or for the family transmission of this disorder. However, clinical, epidemiological and, more recently, experimental evidence indicates that environmental factors have an extraordinary impact on the natural history of type 2 diabetes. Some of these environmental hits are often shared in family groups and proved to be capable to induce epigenetic changes which alter the function of genes affecting major diabetes traits. Thus, epigenetic mechanisms may explain the environmental origin as well as the familial aggregation of type 2 diabetes much easier than common polymorphisms. In the murine model, exposure of parents to environmental hits known to cause epigenetic changes reprograms insulin sensitivity as well as beta-cell function in the progeny, indicating that certain epigenetic changes can be transgenerationally transmitted. Studies from different laboratories revealed that, in humans, lifestyle intervention modulates the epigenome and reverts environmentally induced epigenetic modifications at specific target genes. Finally, specific human epigenotypes have been identified which predict adiposity and type 2 diabetes with much greater power than any polymorphism so far identified. These epigenotypes can be recognized in easily accessible white cells from peripheral blood, indicating that, in the future, epigenetic profiling may enable effective type 2 diabetes prediction. This review discusses recent evidence from the literature supporting the immediate need for further investigation to uncover the power of epigenetics in the prediction, prevention and treatment of type 2 diabetes.



Similar content being viewed by others
References
Kirchner H, Osler ME, Krook A, Zierath JR (2013) Epigenetic flexibility in metabolic regulation: disease cause and prevention? Trends Cell Biol 23(5):203–209
Schwenk RW, Vogel H, Schürmann A (2013) Genetic and epigenetic control of metabolic health. Mol Metab 2(4):337–347
Ungaro P, Mirra P, Oriente F, Nigro C, Ciccarelli M, Vastolo V, Longo M, Perruolo G, Spinelli R, Formisano P, Miele C, Beguinot F (2012) Peroxisome proliferator-activated receptor-γ activation enhances insulin-stimulated glucose disposal by reducing ped/pea-15 gene expression in skeletal muscle cells: evidence for involvement of activator protein-1. J Biol Chem 287(51):42951–42961
Ungaro P, Teperino R, Mirra P, Cassese A, Fiory F, Perruolo G, Miele C, Laakso M, Formisano P, Beguinot F (2008) Molecular cloning and characterization of the human PED/PEA-15 gene promoter reveal antagonistic regulation by hepatocyte nuclear factor 4alpha and chicken ovalbumin upstream promoter transcription factor II. J Biol Chem 283(45):30970–30979
Oriente F, Fernandez Diaz LC, Miele C, Iovino S, Mori S, Diaz VM, Troncone G, Cassese A, Formisano P, Blasi F, Beguinot F (2008) Prep1 deficiency induces protection from diabetes and increased insulin sensitivity through a p160-mediated mechanism. Mol Cell Biol 28(18):5634–5645
Oriente F, Iovino S, Cabaro S, Cassese A, Longobardi E, Miele C, Ungaro P, Formisano P, Blasi F, Beguinot F (2011) Prep1 controls insulin glucoregulatory function in liver by transcriptional targeting of SHP1 tyrosine phosphatase. Diabetes 60(1):138–147
Oriente F, Cabaro S, Liotti A, Longo M, Parrillo L, Pagano TB, Raciti GA, Penkov D, Paciello O, Miele C, Formisano P, Blasi F, Beguinot F (2013) PREP1 deficiency downregulates hepatic lipogenesis and attenuates steatohepatitis in mice. Diabetologia 56(12):2713–2722
Szyf M (2007) The dynamic epigenome and its implications in toxicology. Toxicol Sci 100(1):7–23
Cooper ME, El-Osta A (2010) Epigenetics: mechanisms and implications for diabetic complications. Circ Res 107(12):1403–1413
Paneni F, Costantino S, Volpe M, Lüscher TF, Cosentino F (2013) Epigenetic signatures and vascular risk in type 2 diabetes: a clinical perspective. Atherosclerosis 230(2):191–197
Piarulli F, Sartore G, Lapolla A (2013) Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol 50(2):101–110
Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ (2010) Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467(7318):963–966
Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, Hellman A (2012) Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet 21(2):371–383
International Diabetes Federation (2013) IDF diabetes atlas, 6th edn. Brussels, Belgium
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G, 2010 China Noncommunicable Disease Surveillance Group (2013) Prevalence and control of diabetes in Chinese adults. JAMA 310(9):948–959
Billings LK, Florez JC (2010) The genetics of type 2 diabetes: what have we learned from GWAS? Ann NY Acad Sci 1212:59–77
Raciti GA, Nigro C, Longo M, Parrillo L, Miele C, Formisano P, Béguinot F (2014) Personalized medicine and type 2 diabetes: lesson from epigenetics. Epigenomics 6(2):229–238
Zierath JR, Barrès RE (2011) Nutritional status affects the epigenomic profile of peripheral blood cells. Epigenomics 3(3):259–260
Bennett PH (1990) Epidemiology of diabetes mellitus. In: Rifkin H, Porte D Jr (eds) Ellenberg and Rifkin’s diabetes mellitus, 4th edn. Elsevier, New York, pp 363–370
InterAct Consortium, Scott RA, Langenberg C, Sharp SJ, Franks PW, Rolandsson O, Drogan D, van der Schouw YT, Ekelund U, Kerrison ND, Ardanaz E, Arriola L, Balkau B, Barricarte A, Barroso I, Bendinelli B, Beulens JW, Boeing H, de Lauzon-Guillain B, Deloukas P, Fagherazzi G, Gonzalez C, Griffin SJ, Groop LC, Halkjaer J, Huerta JM, Kaaks R, Khaw KT, Krogh V, Nilsson PM, Norat T, Overvad K, Panico S, Rodriguez-Suarez L, Romaguera D, Romieu I, Sacerdote C, Sánchez MJ, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, van der DL A, Wark PA, McCarthy MI, Riboli E, Wareham NJ (2013) The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia 56(1):60–69
Meigs JB, Cupples LA, Wilson PW (2000) Parental transmission of type 2 diabetes: the Framingham offspring study. Diabetes 49(12):2201–2207
Klein BE, Klein R, Moss SE, Cruickshanks KJ (1996) Parental history of diabetes in a population-based study. Diabetes Care 19(8):827–830
Barnett AH, Eff C, Leslie RD, Pyke DA (1981) Diabetes in identical twins. A study of 200 pairs. Diabetologia 20(2):87–93
Knowles NG, Landchild MA, Fujimoto WY, Kahn SE (2002) Insulin and amylin release are both diminished in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 25(2):292–297
Raciti GA, Beguinot F (2015) Epigenetics of T2DM. Diapedia. http://www.diapedia.org/3105513816/rev/3. Accessed 09 Feb 2015
De Jesus DF, Kulkarni RN (2014) Epigenetic modifiers of islet function and mass. Trends Endocrinol Metab 25(12):628–636
Harris MI (1989) Impaired glucose tolerance in the US population. Diabetes Care 12:464–474
Joost HG (2008) Pathogenesis, risk assessment and prevention of type 2 diabetes mellitus. Obes Facts 1(3):128–137
Vigliotta G, Miele C, Santopietro S, Portella G, Perfetti A, Maitan MA, Cassese A, Oriente F, Trencia A, Fiory F, Romano C, Tiveron C, Tatangelo L, Troncone G, Formisano P, Beguinot F (2004) Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol Cell Biol 24(11):5005–5015
Condorelli G, Vigliotta G, Iavarone C, Caruso M, Tocchetti CG, Andreozzi F, Cafieri A, Tecce MF, Formisano P, Beguinot L, Beguinot F (1998) PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 17(14):3858–3866
Condorelli G, Vigliotta G, Trencia A, Maitan MA, Caruso M, Miele C, Oriente F, Santopietro S, Formisano P, Beguinot F (2001) Protein kinase C (PKC)-α activation inhibits PKC-ζ and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes 50(6):1244–1252
Miele C, Raciti GA, Cassese A, Romano C, Giacco F, Oriente F, Paturzo F, Andreozzi F, Zabatta A, Troncone G, Bosch F, Pujol A, Chneiweiss H, Formisano P, Beguinot F (2007) PED/PEA-15 regulates glucose-induced insulin secretion by restraining potassium channel expression in pancreatic beta-cells. Diabetes 56(3):622–633
Dunn GA, Bale TL (2011) Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152(6):2228–2236
Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR (2012) Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15(3):405–411
Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A, Näslund E, Zierath JR (2013) Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep 3(4):1020–1027
Rönn T, Volkov P, Davegårdh C, Dayeh T, Hall E, Olsson AH, Nilsson E, Tornberg A, Dekker Nitert M, Eriksson KF, Jones HA, Groop L, Ling C (2013) A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet 9(6):e1003572
Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, Emerald BS, Gale CR, Inskip HM, Cooper C, Hanson MA (2011) Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 60(5):1528–1534
Ling C, Del Guerra S, Lupi R, Rönn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51(4):615–622
Barrès R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, Krook A, Zierath JR (2009) Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10(3):189–198
Yang BT, Dayeh TA, Kirkpatrick CL, Taneera J, Kumar R, Groop L, Wollheim CB, Nitert MD, Ling C (2011) Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia 54(2):360–367
Yang BT, Dayeh TA, Volkov PA, Kirkpatrick CL, Malmgren S, Jing X, Renström E, Wollheim CB, Nitert MD, Ling C (2012) Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 26(7):1203–1212
Liu ZH, Chen LL, Deng XL, Song HJ, Liao YF, Zeng TS, Zheng J, Li HQ (2012) Methylation status of CpG sites in the MCP-1 promoter is correlated to serum MCP-1 in type 2 diabetes. J Endocrinol Invest 35(6):585–589
Stepanow S, Reichwald K, Huse K, Gausmann U, Nebel A, Rosenstiel P, Wabitsch M, Fischer-Posovszky P, Platzer M (2011) Allele-specific, age-dependent and BMI-associated DNA methylation of human MCHR1. PLoS One 6(5):e17711
Souren NY, Tierling S, Fryns JP, Derom C, Walter J, Zeegers MP (2011) DNA methylation variability at growth-related imprints does not contribute to overweight in monozygotic twins discordant for BMI. Obesity 19(7):1519–1522
Kuehnen P, Mischke M, Wiegand S, Sers C, Horsthemke B, Lau S, Keil T, Lee YA, Grueters A, Krude H (2012) An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. PLoS Genet 8(3):e1002543
Zhao J, Goldberg J, Vaccarino V (2013) Promoter methylation of serotonin transporter gene is associated with obesity measures: a monozygotic twin study. Int J Obes 37(1):140–145
Groom A, Potter C, Swan DC, Fatemifar G, Evans DM, Ring SM, Turcot V, Pearce MS, Embleton ND, Smith GD, Mathers JC, Relton CL (2012) Postnatal growth and DNA methylation are associated with differential gene expression of the TACSTD2 gene and childhood fat mass. Diabetes 61(2):391–400
Relton CL, Groom A, St Pourcain B, Sayers AE, Swan DC, Embleton ND, Pearce MS, Ring SM, Northstone K, Tobias JH, Trakalo J, Ness AR, Shaheen SO, Smith GD (2012) DNA methylation patterns in cord blood DNA and body size in childhood. PLoS One 7(3):e31821
Drong AW, Lindgren CM, McCarthy MI (2012) The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 92(6):707–715
Acknowledgments
This work has been supported, in part, by the European Foundation for the Study of Diabetes (EFSD), the Associazione Italiana per la Ricerca sul Cancro (AIRC) and by the Ministero dell’Università e della Ricerca Scientifica (Grants PRIN and FIRB-MERIT, and PON 01_02460). This work was also supported by the P.O.R. Campania FSE 2007-2013, Project CREMe.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Statement of human rights
This article does not contain any studies with human participants performed by any of the authors.
Statement on the welfare of animals
All animal procedures in studies conducted by the authors and cited in this review were in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication no. 85-23, revised 1996), and experiments were approved by the ethics committee of the Federico II University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Raciti, G.A., Longo, M., Parrillo, L. et al. Understanding type 2 diabetes: from genetics to epigenetics. Acta Diabetol 52, 821–827 (2015). https://doi.org/10.1007/s00592-015-0741-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0741-0