Abstract
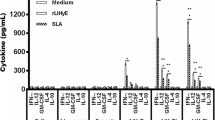
We used TSA as a principal antigen of Leishmania major with adjuvants. The aim of this research was to assess the efficacy of protein vaccine in the presence of Montanide vis-à-vis chitosan nanoparticle. The expression of recombinant protein was confirmed with SDS (sodium dodecyl sulfate) page and Western blotting. A total of 36 BALB/c mice were divided into six groups (TSA/high chitosan, TSA/low chitosan, TSA/Montanide, high chitosan, low chitosan, and PBS groups) and subcutaneously immunized with 20 mg of vaccine at three time intervals on days 0, 14, and 28. The mice were challenged with parasites 21 days after the final immunization. For assaying lymphocyte proliferation, the Brdu test and for evaluation of IgG subclasses and cytokines, the ELISA method was carried out. No statistically noteworthy variation was found between the vaccinated groups before and after the infectious challenge or intervention in terms of IgG and IFN-γ values. Statistically, significant difference was seen among the vaccinated and control groups in terms of IgG and IFN-γ. In terms of IL-4 before and after intervention, no statistically substantial difference was noted in the vaccinated and control groups, whereas, IgG isotypes rose to a great extent prior to and following intervention in all vaccine-immunized groups as compared with control groups. The formulated recombinant TSA protein vaccine with adjuvants induced lymphocytes proliferation and increased cytokines and antibodies as compared with the control groups. Given their potency, the formulated candidate vaccines may be recommended for further studies.







Similar content being viewed by others
References
Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi KA (2004) Comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L.major. Vaccine 22:1631–1639
Askarizadeh A, Jaafari MR, Khamesipour A, Badiee A (2017) Liposomal adjuvant development for leishmaniasis vaccines. Ther Adv Vaccines 5(4–5):85–101
Doavi T, Mousavi SL, Kamali M, Amani J, Fasihi Ramandi M (2016) Chitosan based intranasal vaccine against Escherichia coli.O157:H7. Iran Biomed J 20(2):97–108
Gebreyohannes EA, Bhagvathula AS, Abegaz TM, Seid MA (2018) Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis Poverty 7(1):108. https://doi.org/10.1186/s40249-018-0491-7
Ghorbani M, Farhoudi R (2018) Leishmaniasis in humans: drug or vaccine therapy? Drug Des Dev Ther 12:25–40
Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME (2015) Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 34(26):2992–2995. https://doi.org/10.1016/j.vaccine;.12.071
Hobernik D, Bros M (2018) DNA vaccines—how far from clinical use? Int J Mol Sci 19(11):3605. https://doi.org/10.3390/ijms19113605
Ibrahim HM, El Bisi MK, Taha GM, El Alfy EA (2015) Chitosan nanoparticles loaded antibiotics as drug delivery biomaterial. J Appl Pharm Sci 5(10):85–90
Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV (2010) Selvamurugan N chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym 79:1–8
Lai W-F, Chia-Mi Lin M (2009) Nucleic acid delivery with chitosan and its derivatives. JCR 134:158–168
Li T, Na R, Yu P, Shi B, Yan S, Zhao Y, Xu Y (2015) Effects of dietary supplementation of chitosan on immune and antioxidative function in beef cattle. Czech J Anim Sci 60(1):38–44. https://doi.org/10.17221/7910-CJAS
Liang HF, Chen CT, Chen SC, Kulkarni AR, Chiu YL, Chen MC et al (2006) Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials 27(9):2051–2059
Lone NA, Spackman E, Kapczynski D (2017) Immunologic evaluation of 10 different adjuvants for use in vaccines for chickens against highly pathogenic avian influenza virus. Vaccine 26(8):3401–3408
López-León T, Carvalho EL, Seijo B, Ortega-Vinuesa JL, Bastos-González D (2005) Physicochemical characterization of chitosan nanoparticles: electrokinetic andstability behavior. J Colloid Interface Sci 283:344–351
Mohamed FH, El-sisi AF, Ismail sA, Ismail SA, Hashem AM (2018) The potentiality of using chitosan and its enzymatic depolymerized derivative chito-oligosaccharides as immunomodulators. JAPS 8(12):132–139
Moura MR, Aouada FA, Mattoso LH (2008) Preparation of chitosan nanoparticles using methacrylic acid. J Colloid Interface Sci 321:477–483
Reed SG, Coler RN, Mondal D, Kamhawi S, Valenzuela JG (2016) Leishmania vaccine development: exploiting the host-vector-parasite interface. Expert Rev Vaccines 15(1):81–90. https://doi.org/10.1586/14760584.2016.1105135
Tabatabaie F, Mahdavi M, Faezi S, Dalimi A, Sharifi Z, Akhlaghi L et al (2014) Th1 platform immune responses against Leishmania major induced by thiol-specific antioxidant-based DNA vaccines. Jundishapur J Microbiol 7:e8974
Tabatabaie F, Samarghandi N, Zarrati S, Maleki F, Shafiee Ardestani M et al (2018) Induction of immune responses by DNA vaccines formulated with dendrimer and poly (methyl methacrylate) (PMMA) NanoAdjuvants in BALB/c mice infected with Leishmania major. Maced J Med Sci 6(2):229–235
Tehrani NK, Mahdavi M, Maleki F, Zarrati S, Tabatabaie F (2014) The role of Montanide ISA 70 as an adjuvant in immune responses against Leishmania major induced by thiol-specific antioxidant-based protein vaccine. J Parasit Dis 40:760–767. https://doi.org/10.1007/s12639-014-0574-8
Wei Li X, Ken Lem Lee D, Sun Chi Chan A, Alpar HO (2003) Sustained expression in mammalian cells with DNA complexed with chitosan nanoparticles. BBA 1630:7–18
Zarrati S, Mahdavi M, Tabatabaie F (2014) Immune responses in DNA vaccine formulated with PMMA following immunization and after challenge with Leishmania major. J Parasit Dis 40:427–435. https://doi.org/10.1007/s12639-014-0521-8
Zhao K, Shib X, Zhao Y, Wei H, Sun Q, Huang T et al (2011a) Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 29:8549–8556
Zhao L-M, Shi L-E, Zhang Z-L, Chen J-M, Shi D-D, Yang J, Tang ZX (2011b) Preparation and application of chitosan nanoparticles and nanofibers. Braz J Chem Eng 28:353–362
Funding
This study was financially supported by the Iran University of Medical Sciences (Code: 90-04-30-15896).
Author information
Authors and Affiliations
Contributions
Narges Khabazzade Tehrani and Somayeh Zarrati searched the literature and performed the experiments. Fatemeh Tabatabaie, Fatemeh Maleki, Mehdi Mahdavi, and Abbas ali Imani fooladi designed the study and analyzed the data. Fatemeh Tabatabaie and Mehdi Shafiee Ardestani have participated in drafting the manuscript and supervised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The procedures of this study were also approved by the Ethical Committee of the Faculty of Medicine, Iran University of Medical Sciences, Code: IR.IUMS.REC 90-04-30-15896 in accordance with the guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages: Montanide ISA 70 and chitosan adjuvants are able to improve the effectiveness of a protein vaccine–encoding TSA versus L. major via inducing lymphocytes proliferation as well as increasing cytokines and antibodies in comparison to the control groups.
Rights and permissions
About this article
Cite this article
Maleki, F., Mahdavi, M., Zarrati, S. et al. Induction of immune responses by protein vaccines formulated with adjuvants against Leishmania major in vivo. Comp Clin Pathol 28, 1609–1615 (2019). https://doi.org/10.1007/s00580-019-02976-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-02976-1