Abstract
Purpose
Lidocaine microspheres can prolong the analgesic time to 24–48 h, which still cannot meet the need of postoperative analgesia lasting more than 3 days. Therefore, we added Fe3O4 to the lidocaine microspheres and used an applied magnetic field to attract Fe3O4 to fix the microspheres around the target nerves, reducing the diffusion of magnetic lidocaine microspheres to the surrounding tissues and prolonging the analgesic time.
Methods
Fe3O4–lidocaine–PLGA microspheres were prepared by the complex-emulsion volatilization method to characterize and study the release properties in vitro. The neural anchoring properties and in vivo morphology of the drug were obtained by magnetic resonance imaging. The nerve blocking effect and analgesic effect of magnetic lidocaine microspheres were evaluated by animal experiments.
Results
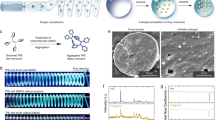
The mean diameter of magnetically responsive lidocaine microspheres: 9.04 ± 3.23 μm. The encapsulation and drug loading of the microspheres were 46.18 ± 3.26% and 6.02 ± 1.87%, respectively. Magnetic resonance imaging showed good imaging of Fe3O4–Lidocain–PLGA microspheres, a drug-carrying model that slowed down the diffusion of the microspheres in the presence of an applied magnetic field. Animal experiments demonstrated that this preparation had a significantly prolonged nerve block, analgesic effect, and a nerve anchoring function.
Conclusion
Magnetically responsive lidocaine microspheres can prolong analgesia by slowly releasing lidocaine, which can be immobilized around the nerve by a magnetic field on the body surface, avoiding premature diffusion of the microspheres to surrounding tissues and improving drug targeting.






Similar content being viewed by others
Data availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, CZ, upon reasonable request.
Abbreviations
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PVA:
-
Polyvinyl alcohol
- DCM:
-
Dichloromethane
- PBS:
-
Phosphate-buffered saline
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- UV:
-
Ultraviolet
- CTMR:
-
Cutaneous trunk reflex
- MRI:
-
Magnetic resonance imaging
References
Coppens SJR, Zawodny Z, Dewinter G, Neyrinck A, Balocco AL, Rex S. In search of the Holy Grail: poisons and extended release local anesthetics. Best Pract Res Clin Anaesthesiol. 2019;33(1):3–21.
Ayyanaar S, Kesavan MP. Magnetic iron oxide nanoparticles@lecithin/poly (l-lactic acid) microspheres for targeted drug release in cancer therapy [published online ahead of print, 2023 Oct 18]. Int J Biol Macromol. 2023;253(Pt 7):127480.
Ben-Akiva E, Karlsson J, Hemmati S, Yu H, Tzeng SY, Pardoll DM, Green JJ. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc Natl Acad Sci U S A. 2023;120(26):e2301606120.
Li H, Zhang X, Zhao W, Cai F, Qin J, Tian J. Efficacy of CalliSpheres® microspheres versus conventional transarterial chemoembolization in the treatment of refractory colorectal cancer liver metastasis. BMC Cancer. 2023;23(1):970.
Korde BA, Mankar JS, Phule S, Krupadam RJ. Nanoporous imprinted polymers (nanoMIPs) for controlled release of cancer drug. Mater Sci Eng C Mater Biol Appl. 2019;99:222–30.
Wang C, Zeng Y, Chen KF, Lin J, Yuan Q, Jiang X, Wu G, Wang F, Jia YG, Li W. A self-monitoring microneedle patch for light-controlled synergistic treatment of melanoma. Bioact Mater. 2023;27:58–71.
Kumbhar PS, Manjappa AS, Shah RR, Nadaf SJ, Disouza JI. Nanostructured lipid carrier-based gel for repurposing simvastatin in localized treatment of breast cancer: formulation design, development, and in vitro and in vivo characterization. AAPS PharmSciTech. 2023;24(5):106.
Zhou L, Feng W, Mao Y, Chen Y, Zhang X. Nanoengineered sonosensitive platelets for synergistically augmented sonodynamic tumor therapy by glutamine deprivation and cascading thrombosis. Bioact Mater. 2022;24:26–36.
Xu X, Chang S, Zhang X, Hou T, Yao H, Zhang S, Zhu Y, Cui X, Wang X. Fabrication of a controlled-release delivery system for relieving sciatica nerve pain using an ultrasound-responsive microcapsule. Front Bioeng Biotechnol. 2022;10:1072205.
Xu Q, Chin SE, Wang CH, Pack DW. Mechanism of drug release from double-walled PDLLA(PLGA) microspheres. Biomaterials. 2013;34(15):3902–11.
Hsu YH, Chen DW, Li MJ, Yu YH, Chou YC, Liu SJ. Sustained delivery of analgesic and antimicrobial agents to knee joint by direct injections of electrosprayed multipharmaceutical-loaded nano/microparticles. Polymers (Basel). 2018;10(8):890.
Guo Y, Logan HL, Glueck DH, Muller KE. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol. 2013;13:100.
Lipengolts AA, Finogenova YA, Skribitsky VA, Shpakova KE, Anaki A, Motiei M, Semkina AS, Abakumov MA, Smirnova AV, Grigorieva EY, Popovtzer R. CT and MRI Imaging of Theranostic Bimodal Fe3O4@Au NanoParticles in Tumor Bearing Mice. Int J Mol Sci. 2022;24(1):70.
Yamasaki T, Fujiwara H, Oda R, Mikami Y, Ikeda T, Nagae M, Shirai T, Morisaki S, Ikoma K, Masugi-Tokita M, Yamada K. In vivo evaluation of rabbit sciatic nerve regeneration with diffusion tensor imaging (DTI): correlations with histology and behavior. Magn Reson Imaging. 2015;33(1):95–101.
Chou AK, Chiu CC, Chen YW, Wang JJ, Hung CH. Phentolamine reverses epinephrine-enhanced skin antinociception of dibucaine in rats. Anesth Analg. 2019;128(6):1336–43.
Tzeng JI, Wang JN, Wang JJ, Chen YW, Hung CH. Cutaneous synergistic analgesia of bupivacaine in combination with dopamine in rats. Neurosci Lett. 2016;620:88–92.
Wang J, Helder L, Shao J, Jansen JA, Yang M, Yang F. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: effect of polymer end groups. Int J Pharm. 2019;564:1–9.
Malpezzi-Marinho ELA, Zanoni CIS, Molska GR, Paraventi C, Wuo-Silva R, Berro LF, Parada CA, Tamura EK, Marinho EA. Antinociceptive Activity of the Skin Secretion of Phyllomedusa rohdei (Amphibia, Anura). Toxins (Basel). 2020;12(9):589.
Kau YC, Liao CC, Chen YC, Liu SJ. Sustained release of lidocaine from solvent-free biodegradable poly[(d,l)-Lactide-co-Glycolide] (PLGA): in vitro and in vivo study. materials (Basel). 2014;7(9):6660–76.
Zhang X, Dang M, Zhang W, Lei Y, Zhou W. Sustained delivery of prilocaine and lidocaine using depot microemulsion system: in vitro, ex vivo and in vivo animal studies. Drug Dev Ind Pharm. 2020;46(2):264–71.
Svirskis D, Chandramouli K, Bhusal P, Wu Z, Alphonso J, Chow J, Patel D, Ramakrishna R, Yeo SJ, Stowers R, Hill A. Injectable thermosensitive gelling delivery system for the sustained release of lidocaine [published correction appears in Ther Deliv. 2016;7(7):511]. Ther Deliv. 2016;7(6):359–68.
Bnyan R, Khan I, Ehtezazi T, Saleem I, Gordon S, O’Neill F, Roberts M. Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic. Pharm Pharmacol. 2019;71(10):1508–19.
Wen K, Na X, Yuan M, Bazybek N, Li X, Wei Y, Ma G. Preparation of novel ropivacaine hydrochloride-loaded PLGA microspheres based on post-loading mode and efficacy evaluation. Colloids Surf B Biointerfaces. 2022;210:112215.
Essa D, Kondiah PPD, Choonara YE, Pillay V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front Bioeng Biotechnol. 2020;8:48.
Su Y, Liu J, Tan S, Liu W, Wang R, Chen C. PLGA sustained-release microspheres loaded with an insoluble small-molecule drug: microfluidic-based preparation, optimization, characterization, and evaluation in vitro and in vivo. Drug Deliv. 2022;29(1):1437–46.
Zhu W, Chen L, Wu Z, Li W, Liu X, Wang Y, Guo M, Ito Y, Wang L, Zhang P, Wang H. Bioorthogonal DOPA-NGF activated tissue engineering microunits for recovery from traumatic brain injury by microenvironment regulation. Acta Biomater. 2022;150:67–82.
Manto KM, Govindappa PK, Martinazzi B, Han A, Hegarty JP, Koroneos Z, Talukder MH, Elfar JC. Erythropoietin-PLGA-PEG as a local treatment to promote functional recovery and neurovascular regeneration after peripheral nerve injury. J Nanobiotechnol. 2022;20(1):461.
Yu M, Yuan W, Xia Z, Liu Y, Wang Y, Xu X, Zheng J, Schwendeman A. Characterization of exparel bupivacaine multivesicular liposomes. Int J Pharm. 2023;639:122952.
Blair HA. Bupivacaine/meloxicam prolonged release: a review in postoperative pain. Drugs. 2021;81(10):1203–11.
Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2019;16(1):69–78.
Zhao S, Yu X, Qian Y, Chen W, Shen J. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics. 2020;10(14):6278–309.
Wainwright TW, Gill M, Mcdonald DA, Middleton RG, Reed M, Sahota O, Yates P, Ljungqvist O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020;91(1):3–19.
Frassanito L, Vergari A, Nestorini R, Cerulli G, Placella G, Pace V. Enhanced recovery after surgery (ERAS) in hip and knee replacement surgery: description of a multidisciplinary program to improve management of the patients undergoing major orthopedic surgery. Musculoskelet Surg. 2020;104(1):87–92.
Chen Q, Ma X, Xie L, Chen W, Xu Z, Song E, Zhu X, Song Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale. 2021;13(9):4855–70.
Tousi MS, Sepehri H, Khoee S, Farimani MM, Delphi L, Mansourizadeh F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J Pharm Anal. 2021;11(1):108–21.
Peng F, Liu J, Zhang Y, Fan J, Gong D, He L, Zhang W, Qiu F. Designer self-assembling peptide nanofibers induce biomineralization of lidocaine for slow-release and prolonged analgesia. Acta Biomater. 2022;146:66–79.
Acknowledgements
This work was supported by grants from the Medical Science and Technology Project of Sichuan Provincial Health Commission (21PJ142) and the Chengdu Municipal Health Commission (2022167).
Funding
Health Commission of Sichuan Province, 21PJ142, Chuandong Zheng.
Author information
Authors and Affiliations
Contributions
LZ: This author helped conduct the experiments, analyze the statistics and write the article. QY: This author helped perform experiments. QL: This author helped perform experiments. CZ: This author helped design the study and provided experimental guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
540_2023_3305_MOESM2_ESM.jpg
Supplementary file2 CTMR response inhibition rate and number of CTMR unprimed in each group. Two-way analysis of variance (two-way ANOVA) and Tukey’s test were used to analyze the results of each group at each time. Statistical significance was shown: ***P < 0.001(between M1 and NM1); ****P < 0.0001(between M1 and NM1); ####P < 0.0001(between M1 and S1); &&&&P < 0.0001(between NM1 and S1); &&&P < 0.001(between NM1 and S1) (JPG 1870 KB)
540_2023_3305_MOESM3_ESM.jpg
Supplementary file3 Pain threshold of rats in each group. Two-way ANOVA and Tukey’s test were used to analyze the results of each group at each time. Statistical significance was shown: *P < 0.05(between M2 and NM2); ***P < 0.001(between M2 and NM2), ****P < 0.0001(between M2 and NM2); #P < 0.05(between M2 and S2) ####P < 0.0001(between M2 and S2); &&&&P < 0.0001(between NM2 and S2) (JPG 728 KB)
About this article
Cite this article
Zheng, Lx., Yu, Q., Li, Q. et al. Targeted local anesthesia: a novel slow-release Fe3O4–lidocaine–PLGA microsphere endowed with a magnetic targeting function. J Anesth 38, 232–243 (2024). https://doi.org/10.1007/s00540-023-03305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-023-03305-1