Abstract
Background
Spermidine suppress oxidative stress and is involved in various disease pathogenesis including ulcerative colitis (UC). Arginase 2 (ARG2) plays a central role in the synthesis of spermidine. This study aimed to clarify the effect of endogenously produced spermidine on colitis.
Methods
The physiological role of ARG2 and spermidine was investigated using Arg2-deficient mice with reduced spermidine. Immunohistochemical staining of the rectum was used to analyze ARG2 expression and spermidine levels in healthy controls and UC patients.
Results
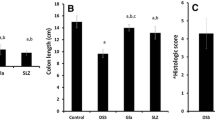
In mice with dextran sulfate sodium-induced colitis, ARG2 and spermidine levels were increased in the rectal epithelium. Spermidine protects colonic epithelial cells from oxidative stress and Arg2 knockdown cells reduced antioxidant activity. Organoids cultured from the small intestine and colon of Arg2-deficient mice both were more susceptible to oxidative stress. Colitis was exacerbated in Arg2-deficient mice compared to wild-type mice. Supplementation with spermidine result in comparable severity of colitis in both wild-type and Arg2-deficient mice. In the active phase of UC, rectal ARG2 expression and spermidine accumulation were increased compared to remission. ARG2 and spermidine levels were similar in healthy controls and UC remission patients.
Conclusions
ARG2 produces spermidine endogenously in the intestinal epithelium and has a palliative effect on ulcerative colitis. ARG2 and spermidine are potential novel therapeutic targets for UC.












Similar content being viewed by others
Abbreviations
- ARG2:
-
Arginase 2
- DAPI:
-
4,6-Diamino-2-phenylindole
- DSS:
-
Dextran sulfate sodium
- GCL:
-
Glutamate–cysteine ligase
- GCLC:
-
Glutamate–cysteine ligase catalytic subunit
- GCLM:
-
Glutamate–cysteine ligase modifier subunit
- HDAC2:
-
Histone deacetylase 2
- HE:
-
Hematoxylin–eosin
- HO-1:
-
Heme oxygenase-1
- H2O2 :
-
Hydrogen peroxide
- IBD:
-
Inflammatory bowel disease
- KO:
-
Knockout
- LPS:
-
Lipopolysaccharide
- NQO1:
-
Nicotinamide quinone oxidoreductase 1
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- PCR:
-
Polymerase chain reaction
- ROS:
-
Reactive oxygen species
- SPD:
-
Spermidine
- TNFα:
-
Tumor necrosis factor-α
- TXNR:
-
Thioredoxin reductase 1
- UC:
-
Ulcerative colitis
References
Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74.
Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78.
Nakase H, Sato N, Mizuno N, et al. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022;21: 103017.
Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(1324–35): e2.
Ihara Y, Torisu T, Miyawaki K, et al. Ustekinumab improves active Crohn’s disease by suppressing the T helper 17 pathway. Digestion. 2021;102:946–55.
Imazu N, Torisu T, Ihara Y, et al. Ustekinumab decreases circulating Th17 cells in ulcerative colitis. Intern Med. 2023;63:153.
Madeo F, Hofer SJ, Pendl T, et al. Nutritional aspects of spermidine. Annu Rev Nutr. 2020;40:135–59.
Zou D, Zhao Z, Li L, et al. A comprehensive review of spermidine: safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Compr Rev Food Sci Food Saf. 2022;21:2820–42.
Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14.
Timmons J, Chang ET, Wang JY, et al. Polyamines and gut mucosal homeostasis. J Gastrointest Dig Syst. 2012. https://doi.org/10.4172/2161-069X.S7-001.
Weiss TS, Herfarth H, Obermeier F, et al. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:529–35.
Obayashi M, Matsui-Yuasa I, Matsumoto T, et al. Polyamine metabolism in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol. 1992;87:736–40.
Gobert AP, Latour YL, Asim M, et al. Protective role of spermidine in colitis and colon carcinogenesis. Gastroenterology. 2022;162(813–27): e8.
Nakamura A, Kurihara S, Takahashi D, et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat Commun. 2021;12:2105.
Yu H, Yoo PK, Aguirre CC, et al. Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem. 2003;51:1151–60.
Choi S, Park C, Ahn M, et al. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem. 2012;114:487–94.
Coburn LA, Gong X, Singh K, et al. l-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE. 2012;7: e33546.
Zwintscher NP, Shah PM, Salgar SK, et al. Hepatocyte growth factor, hepatocyte growth factor activator and arginine in a rat fulminant colitis model. Ann Med Surg (Lond). 2016;7:97–103.
Kudo T, Matsumoto T, Nakamichi I, et al. Recombinant human granulocyte colony-stimulating factor reduces colonic epithelial cell apoptosis and ameliorates murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:689–97.
Hara M, Torisu K, Tomita K, et al. Arginase 2 is a mediator of ischemia-reperfusion injury in the kidney through regulation of nitrosative stress. Kidney Int. 2020;98:673–85.
Sakuma S, Abe M, Kohda T, et al. Hydrogen peroxide generated by xanthine/xanthine oxidase system represses the proliferation of colorectal cancer cell line Caco-2. J Clin Biochem Nutr. 2015;56:15–9.
Kawano S, Torisu T, Esaki M, et al. Autophagy promotes degradation of internalized collagen and regulates distribution of focal adhesions to suppress cell adhesion. Biol Open. 2017;6:1644–53.
Li B, Alli R, Vogel P, et al. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869–78.
Torisu T, Torisu K, Lee IH, et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med. 2013;19:1281–7.
Bajic D, Niemann A, Hillmer AK, et al. Gut microbiota-derived propionate regulates the expression of Reg3 mucosal lectins and ameliorates experimental colitis in mice. J Crohns Colitis. 2020;14:1462–72.
Ren W, Yin J, Wu M, et al. Serum amino acids profile and the beneficial effects of l-arginine or l-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE. 2014;9: e88335.
Andrade ME, Santos RD, Soares AD, et al. Pretreatment and treatment with l-arginine attenuate weight loss and bacterial translocation in dextran sulfate sodium colitis. JPEN J Parenter Enteral Nutr. 2016;40:1131–9.
Singh K, Gobert AP, Coburn LA, et al. Dietary arginine regulates severity of experimental colitis and affects the colonic microbiome. Front Cell Infect Microbiol. 2019;9:66.
Coburn LA, Horst SN, Allaman MM, et al. l-Arginine availability and metabolism is altered in ulcerative colitis. Inflamm Bowel Dis. 2016;22:1847–58.
Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14.
Niechcial A, Schwarzfischer M, Wawrzyniak M, et al. Spermidine ameliorates colitis via induction of anti-inflammatory macrophages and prevention of intestinal dysbiosis. J Crohns Colitis. 2023;17:1489.
Liu P, de la Vega MR, Dodson M, et al. Spermidine confers liver protection by enhancing NRF2 signaling through a MAP1S-mediated noncanonical mechanism. Hepatology. 2019;70:372–88.
Aihara S, Torisu K, Uchida Y, et al. Spermidine from arginine metabolism activates Nrf2 and inhibits kidney fibrosis. Commun Biol. 2023;6:676.
Yan J, Yan JY, Wang YX, et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br J Pharmacol. 2019;176:3126–42.
Xu TT, Li H, Dai Z, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY). 2020;12:6401–14.
Medina CB, Mehrotra P, Arandjelovic S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–5.
Murray Stewart T, Dunston TT, Woster PM, et al. Polyamine catabolism and oxidative damage. J Biol Chem. 2018;293:18736–45.
Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44.
Piotrowska M, Swierczynski M, Fichna J, et al. The Nrf2 in the pathophysiology of the intestine: molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol Res. 2021;163: 105243.
Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–47.
Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–17.
Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87.
Dowling JK, Afzal R, Gearing LJ, et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat Commun. 2021;12:1460.
Geiger R, Rieckmann JC, Wolf T, et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(829–42): e13.
Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465–6.
Rachmilewitz D, Stamler JS, Bachwich D, et al. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut. 1995;36:718–23.
Yasukawa K, Tokuda H, Tun X, et al. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res. 2012;46:1427–36.
Acknowledgements
The authors appreciate the technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University. The authors also thank Mr. M. Munakata and Mrs. M Tanaka at the Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University for assistance with histology. The authors thank Ellen Knapp, PhD, from Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript. The authors acknowledge BioRender.com to create graphic abstract.
Funding
This work was supported by JSPS KAKENHI [Grant No. JP21K06783] and a Grant-in-Aid for Scientific Research C.
Author information
Authors and Affiliations
Contributions
NI, and TT conceived the idea of the study. NI, AY, SK, T. Nitahata, YU, SA, and TT were involved in providing guidance on the animal experiments and data analysis. JU, KK, SF, YF, YM, T. Nagasue, TM, YT, and TT collected the clinical samples and contributed to interpretation of the data. TK, YO, and TT contributed to drafting of the manuscript and critical revision. All authors revised the manuscript, approved the manuscript to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imazu, N., Torisu, T., Yokote, A. et al. Arginase 2 attenuates ulcerative colitis by antioxidant effects of spermidine. J Gastroenterol (2024). https://doi.org/10.1007/s00535-024-02104-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00535-024-02104-z