Abstract
During the natural course of chronic hepatitis B virus infection, immune-tolerant phase is characterized by high viral replication, the presence of HBV e antigen (HBeAg), and normal or minimally elevated serum alanine aminotransferase. Immune-tolerant phase is usually regarded as a benign course of the disease. International guidelines recommend observation rather than treatment during immune-tolerant phase. In this article, we review unresolved issues related to the definition of true immune-tolerant phase and the benefit of antiviral treatment. Defining true immune-tolerant phase requires a careful approach and long-term follow-up. In previous studies, many patients were misclassified as being immune-tolerant phase. Noninvasive methods of assessing fibrosis are warranted for patients in the immune-tolerant phase. Yet, there has been controversy over the benefit and harm of antiviral treatment for immune-tolerant phase patients. Thus, further larger scale studies are needed to investigate the prognosis of patients in true immune-tolerant phase and their need for antiviral therapy.
Similar content being viewed by others
Current understanding of immune-tolerant phase
Hepatitis B virus (HBV) infection is one of the most common liver diseases worldwide. Chronic HBV infection can progress to cirrhosis or hepatocellular carcinoma (HCC). In Asia, patients with chronic hepatitis B (CHB) mostly acquire HBV infection by perinatal transmission or during infancy [1]. The majority of neonates or children exposed to HBV develop chronic infection and enter a prolonged immune-tolerant phase [1].
In perinatal acquired HBV infection, it usually comprises four phases: immune-tolerant phase, immune-clearance or active phase, inactive-carrier phase, and reactivation phase [2]. The earliest phase is characterized by very high viral replication, the presence of HBV e antigen (HBeAg), and normal or minimally elevated serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) [3]. This immune-tolerant phase is typically seen in patients younger than age 30 [4, 5]. The immune-clearance phase, which occurs during adolescence or adulthood, is accompanied by continuing hepatitis activity or episodic hepatitis flares. These might develop to fibrosis or cirrhosis during the HBeAg-positive phase or result in declining serum HBV DNA and HBeAg seroconversion. After HBeAg seroconversion, most patients enter the ‘inactive’ phase, which is characterized by sustained normal serum ALT and low HBV DNA [6]. However, hepatitis may relapse because of reactivation of HBV with either HBeAg seroreversion or development of precore or basal core prompter mutations. The immunopathogenesis of HBeAg-negative hepatitis is similar to that of HBeAg-positive hepatitis. Therefore, this phase is considered a variant of immune-clearance phase [7].
HBeAg is a small immunogenic secretory viral protein. HBeAg can pass through the placenta and induce clonal deletion of T cells against HBV in the fetus [8]. When the infection is acquired at birth, viral replication is usually very high, but hepatic damage is minimal. Liver biopsy typically shows no fibrosis and minimal inflammation. This can be explained by the relative lack of immune pressure on the virus [9]. Early natural history studies of immune-tolerant phase revealed a benign disease course. A study using paired liver biopsy data showed minimal progression of liver injury over 5 years among patients who remained in the immune-tolerant phase [10]. Three (6.3%, 3/48) patients had fibrosis progression and four (12.1%, 4/33) patients of F1 at baseline regressed to F0. Thus, international guidelines recommend observation rather than active treatment for patients in the immune-tolerant phase [4, 5, 11].
Challenges on definition of immune-tolerant phase
“Defining true immune-tolerant” phase is challenging. Both natural killer (NK) and T cells are functionally impaired in the immune-tolerant and immune-active phases [12]. In one study, the proportion of NK cells in the liver was significantly higher in patients in the immune-tolerant phase compared to those in the immune-clearance phase [13]. In addition, there might be intrahepatic HBV-specific T cell activities in immune-tolerant-phase patients, even in the absence of liver inflammation [14]. A histology study in India showed that approximately 40% of HBeAg-positive patients with persistently normal ALT had necro-inflammation and fibrosis histologic fibrosis ≥ stage 2 [15]. In a retrospective cohort study in Korea, it was shown that the risk of developing hepatocellular carcinoma (HCC) was higher in untreated patients in the immune-tolerant phase than patients in the immune-active phase on antivirals (12.7% vs. 6.0%, p = 0.001) [16]. One possible reason for the poor outcome of immune-tolerant patients in previous studies is misclassification of patients in immune clearance phase as immune-tolerant. For example, in the Korean study, the mean age of the immune-tolerant patients was 38 years and 26% of immune-tolerant patients had HBV DNA 4–7 log IU/ml, which were atypical features for immune-tolerant phase [16]. In the Indian histology cohort, the lower range HBV DNA among HBeAg-positive patients with persistently normal ALT was only 2.78 log copies/ml, which was too low for immune-tolerant phase [15]. Therefore, defining immune-tolerant phase is challenging, and whether a true immune-tolerant phase exists is debatable. The 2017 European Association for the Study of the Liver (EASL) Clinical Practice Guidelines renamed this phase as HBeAg-positive chronic HBV infection instead of immune-tolerant phase to avoid confusion in terminology [11].
Refining the definition of immune-tolerant using biomarkers
Normal ALT level
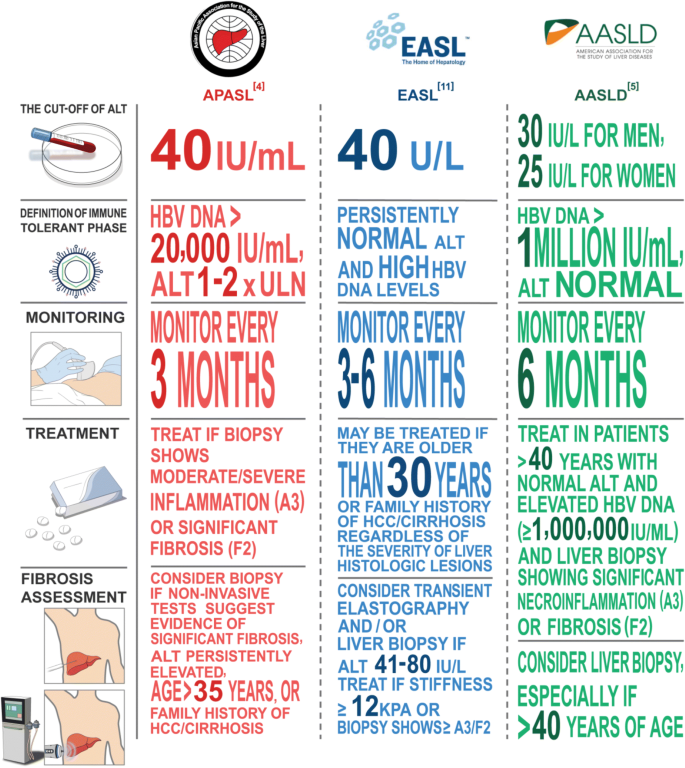
Normal ALT is a key criterion to define immune-tolerant phase. The upper limit of normal (ULN) ALT in healthy subjects is 29–33 U/l for males and 19–25 U/L for females [17,18,19]. The American Association for the Study of Liver Diseases (AASLD) defines normal ALT as < 30 U/l for males and < 25 U/l for females [5] (Fig. 1). The Asian Pacific Association for the Study of the Liver (APASL) and EASL guidelines use the established ALT cutoff of 40 U/L [4, 11]. In the definition of normal ALT, metabolic risk factors for fatty liver disease are generally excluded. Hence a CHB patient in immune-tolerant phase can have elevated ALT if there is co-existing fatty liver disease, which has a prevalence of approximately 29.6% in Asia [20].
On the other hand, some patients with normal ALT can have liver fibrosis, as immune clearance activities might be intermittent and quiet down when ALT is checked. In a prospective study, 10% of HBeAg-positive patients with normal ALT had advanced fibrosis as assessed by transient elastography [21]. The risk for advanced liver fibrosis increases in HBeAg-positive patients older than 35 years with ALT greater than 0.5 times the ULN. Therefore, normal ALT alone in HBeAg-positive patients is insufficient to define immune-tolerant in CHB.
HBV DNA levels
HBV DNA is expected to be very high in patients who have not experienced any immune clearance. In HBeAg-positive asymptomatic children, HBV DNA is in general > 7 log IU/ml [22] [Fig. 1]. The EASL Clinical Practice Guidelines has adopted HBV DNA > 7 log U/ml to define HBeAg-positive chronic infection [11]. AASLD guideline sets a lower bar of HBV DNA > 1 million IU/ml to define immune-tolerant phase, which has a risk of misclassifying some patients in immune-clearance phase as immune-tolerant [23]. Hence one should have a high degree of suspicion that a HBeAg-positive patient with normal ALT is not in true immune-tolerant phase but has stepped into immune clearance when HBV DNA is < 7 log IU/ml.
HBsAg level
Quantification of hepatitis B surface antigen (HBsAg) largely reflects the concentration of covalently closed circular DNA (cccDNA) in the liver in HBeAg-positive patients [24]. HBsAg is translated from mRNA of the transcriptional active template cccDNA, which reflects the number of infected hepatocytes [25]. The mean baseline HBsAg levels differ significantly during different phases of CHB [26]. The HBsAg level is highest in immune-tolerant phase and lowest in liver cirrhosis phase: immune-tolerant (4.53 − 4.96 log10 U/mL), immune-clearance (4.03 − 4.37 log10 IU/mL), e antigen negative hepatitis (2.95 log10 IU/mL), low replicative (3.18 log10 IU/mL), and liver cirrhosis (2.69 log10 IU/mL) [26,27,28]. In one longitudinal cohort study in Hong Kong, HBsAg remained persistently high at approximately 5 log IU/mL in patients of immune-tolerant phase over a period of 8 years [29]. Thus, high HBsAg level may assist differentiation of immune-tolerant from immune-clearance in HBeAg-positive patients [30]. However, no clear HBsAg cutoff value has been identified with high sensitivity and specificity to define immune-tolerant phase.
Liver biopsy and non-invasive assessment: impact of age
When the clinician is not certain if a HBeAg-positive patient is in the immune-tolerant phase, liver biopsy can be performed to detect evidence of necro-inflammation or liver fibrosis, which will trigger commencement of antiviral therapy (Fig. 1). According to the AASLD guideline, liver biopsy should be considered for patients with persistent borderline normal or slightly elevated ALT, in particular those 40 years or older and infected at a young age [31]. The APASL guideline states that liver biopsy should be considered for patients with abnormal transient elastography and older than age 35. The EASL guideline recommends that patients with ALT 41–80 IU/L be evaluated by transient elastography and/or liver biopsy, while antiviral therapy should be considered for patients older than age 30 even without assessment of liver fibrosis. While older age is generally agreed to be a good surrogate of immune-clearance, the cut-off age for immune-tolerant phase is unclear.
As reflected in the guidelines, liver biopsy is invasive and cannot be performed in all cases. There is an increasing need for noninvasive assessments such as transient elastography and/or serum tests. Noninvasive tests might be used to assess for the severity of fibrosis [32, 33]. Patients who have liver stiffness measurement > 8 kPa by transient elastography or APRI > 1.5 might have significant fibrosis. However, clinical application of noninvasive markers has several limitations. The diagnostic accuracy of noninvasive test is sometimes unsatisfactory. For example, AST/platelet ratio index showed moderate diagnostic performance for the assessment of fibrosis in patients with CHB [34]. Although liver stiffness measurement may have a higher diagnostic accuracy than serum noninvasive tests [35, 36], its usefulness may be reduced in obese patients [37]. In general, the specificity of liver stiffness measurement to exclude significant fibrosis in patients with normal ALT is very high [38]. Sequential use of liver stiffness measurement and serum indexes such as Forn’s index or Enhanced Liver Fibrosis test improve the accuracy to predict liver fibrosis [39, 40].
Controversies of antiviral treatment in immune-tolerant patients
The need to treat
There has been controversy over the need to treat patients in immune-tolerant phase. It is apparent that some patients will undergo spontaneous HBeAg seroconversion and may not need antiviral therapy. Patients with spontaneous HBeAg seroconversion before age 30 have an excellent prognosis; the 15-year cumulative incidence of cirrhosis and HCC was 3.7% and 2.1%, respectively [41]. On the other hand, older patients who remain in HBeAg-positive state may have poorer outcome, and some patients might silently move into immune clearance phase with liver injury. In one Korean study, among patients with a high viral load and normal or slightly elevated serum ALT for at least 12 months, 60% had significant fibrosis and 62% showed significant histology [42]. In Hong Kong, among HBeAg-positive patients older than age 35 with ALT greater than 0.5 × the ULN, 37% had advanced fibrosis as assessed by transient elastography [21].
The public health burden of HBV is increased by its lifelong course and the requirement for long-term follow-up [43]. There is a risk of horizontal HBV transmission from immune-tolerant patients with very high viral load. Another issue is vertical transmission of HBV by high viral load mothers. Even with hepatitis B immunoglobulin, timely birth dose and 3-dose vaccination to the newborn, approximately 5–10% of newborns will acquire HBV infection if maternal viral load is higher than 6 log IU/ml [44, 45]. Therefore, international guidelines recommend that HBeAg-positive mothers with HBV DNA > 6 log IU/mL or 200,000 IU/mL should receive oral antivirals during the last trimester, together with hepatitis B immunoglobulin and vaccination for newborns.
Most children and adults will progress from the immune-tolerant phase to the immune-active phase. There is controversy whether the risk of HCC will increase in immune-tolerant patients if they are not treated. One Korean study showed that the 10-year cumulative incidence of HCC was 12.7% among patients in the immune-tolerant phase compared to 6.0% among those in the immune-active phase, though there is concern on possible misclassification of immune-tolerant patients in this study [16]. Another Koreans study showed the contrary; the cumulative risk for HCC was similar in patients in the immune-tolerant phase and those with a virologic response groups to antivirals (1.1% and 2.7% vs. 1.0% and 2.9% at 5 and 10 years, respectively; p = 0.704) [46]. A study reported that suppressing HBV DNA to prevent HCC and cirrhosis is cost-effective in immune-tolerant phase patients [47]. The low cost of antiviral drugs nowadays has lowered the financial barrier for universal coverage of HBV treatment in most countries.
Limitations of antiviral therapy
Patients in immune-tolerant phase are very difficult to treat. Patients in immune-tolerant phase respond poorly to interferon-alpha therapy [48]. In a recent study using 8 weeks of entecavir followed by combination peginterferon and entecavir therapy for 40 weeks in adults in the immune-tolerant phase, only 4% patients had HBeAg seroconversion and 0% patients had HBV DNA < 1000 IU/ml at 48 weeks after completion of peginterferon treatment [49]. A randomized controlled trial of tenofovir disoproxil fumarate (TDF) versus emtricitabine and TDF in HBeAg-positive patients with high HBV DNA (> 7 log IU/mL) and normal ALT showed that HBV DNA can be suppressed in < 60% patients and HBeAg seroconversion developed in < 5% patients in 4 years [49]. After the cessation of TDF-based treatment, all patients experienced HBV DNA relapse to > 2000 IU/mL within 4 weeks, and 50% showed an increase in ALT [50]. As a result, long-term treatment is expected if nucleot(s)ide analogue is used in immune-tolerant patients, and adherence to treatment among young patients will be a concern. Considering the minimal risk for disease progression, anticipated long-term treatment in younger patients, poor response to treatment in patients in the immune-tolerant phase, lack of data on improvement in long-term outcomes, nucleot(s)ide analogue therapy is generally not recommended for patients in immune-tolerant phase of CHB at the present moment [51].
Summary, recommendations, directions for future research
No international consensus has been made to define true immune-tolerant phase and studies on prognosis and treatment response in true immune-tolerant phase are limited. There is no placebo-controlled study on the long-term benefit of antiviral therapy in immune-tolerant phase over observation. Given the low event rate of HCC and requirement of long-term follow-up, a randomized, controlled study to evaluate the benefit of antiviral treatment in immune-tolerant patients seems not very feasible. Most clinicians agree on the need to identify advanced fibrosis and appropriate treatment in HBeAg-positive patients with a high viral load and a normal ALT level for older patients, but the cut-off age for detailed assessment is debatable. In a true immune-tolerant phase, the risk for disease progression is minimal. Even after antiviral treatment is started, the virological response is unsatisfactory and off-treatment relapse often occurs. Thus, further studies are needed to determine the assessment and treatment strategy for immune-tolerant patients.
Abbreviations
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- CHB:
-
Chronic hepatitis B
- HBeAg:
-
Hepatitis B e antigen
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- NK:
-
Natural killer
- EASL:
-
European Association for the Study of the Liver
- ULN:
-
Upper limit of normal
- AASLD:
-
American Association for the Study of Liver Disease
- APASL:
-
Asian Pacific Association for the Study of the Liver
- HBsAg:
-
Hepatitis B surface antigen
- cccDNA:
-
Covalently closed circular DNA
- qHBSAg:
-
Quantitative HBsAg
- LSM:
-
Liver stiffness measurement
- WHO:
-
World Health Organization
- TDF:
-
Tenofovir disoproxil fumarate
References
Chu C, Liaw Y. Natural history differences in perinatally versus adult-acquired disease. Curr Hepat Rep. 2004;3:123–31.
Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63.
Lai M, Hyatt BJ, Nasser I, et al. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–7.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–7.
Liaw Y-F, Chu C-M. Hepatitis B virus infection. The Lancet. 2009;373:582–92.
Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075–86.
Chan HLY. Okuda lecture: challenges of hepatitis B in the era of antiviral therapy. J Gastroenterol Hepatol. 2019;34:501–6.
Hui CK, Leung N, Yuen ST, et al. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395–401.
EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–98.
Wang WT, Zhao XQ, Li GP, et al. Immune response pattern varies with the natural history of chronic hepatitis B. World J Gastroenterol. 2019;25:1950–63.
Sprengers D, van der Molen RG, Kusters JG, et al. Different composition of intrahepatic lymphocytes in the immune-tolerance and immune-clearance phase of chronic hepatitis B. J Med Virol. 2006;78:561–8.
Kennedy PTF, Sandalova E, Jo J, et al. Preserved T cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–45.
Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–84.
Kim GA, Lim YS, Han S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945–52.
Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10.
Lee JK, Shim JH, Lee HC, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577–83.
Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55:447–54.
Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–98.
Wong GL, Wong VW, Choi PC, et al. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227–33.
Lok AS, Lai CL. A longitudinal follow-up of asymptomatic hepatitis B surface antigen-positive Chinese children. Hepatology. 1988;8:1130–3.
Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83.
Chan HL, Wong VW, Tse AM, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–8.
Martinot-Peignoux M, Lapalus M, Asselah T, et al. HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int. 2014;34(Suppl 1):97–107.
Zeng LY, Lian JS, Chen JY, et al. Hepatitis B surface antigen levels during natural history of chronic hepatitis B: a Chinese perspective study. World J Gastroenterol. 2014;20:9178–84.
Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508–13.
Martinot-Peignou M, Lapalus M, Lada O, et al. Natural history of hepatitis B virus (HBV) infection: role Of HBV genotypes (A TO E) assessed in a large cohort: 506. Hepatology. 2011;54(suppl 1):609A.
Chan HL, Wong VW, Wong GL, et al. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52:1232–41.
Cornberg M, Wong VW, Locarnini S, et al. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398–411.
Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147–52.
Cheng J, Hou J, Ding H, et al. Validation of ten noninvasive diagnostic models for prediction of liver fibrosis in patients with chronic hepatitis B. PLoS ONE. 2015;10:e0144425.
Jia J, Hou J, Ding H, et al. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756–62.
Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302.
EASL-ALEH Clinical Practice Guidelines. Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64.
Wong GL, Wong VW, Chim AM, et al. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol. 2011;26:300–5.
Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811–9.
Chan HL, Wong GL, Choi PC, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44.
Wong GL, Chan HL, Choi PC, et al. Non-invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2014;39:197–208.
Wong GL, Wong VW, Choi PC, et al. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2010;31:1095–103.
Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435–44.
Park JY, Park YN, Kim DY, et al. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with genotype C and high serum HBV DNA levels. J Viral Hepat. 2008;15:615–21.
Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49:S4–S12.
Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324–34.
Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911–23.
Lee HW, Kim SU, Oidov B, et al. Comparison between chronic hepatitis B patients with untreated immune-tolerant phase vs those with virological response by antivirals. Sci Rep. 2019;9:2508.
Enriquez A, Campbell M, Reddy K. Cost-effectiveness of suppressing hepatitis B virus DNA in immune tolerant patients to prevent hepatocellular carcinoma and cirrhosis. Aliment Pharmacol Ther. 2007;26:383–91.
Lok AS, Wu P-C, Lai C-L, et al. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992;102:2091–7.
Chan HL, Chan CK, Hui AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology. 2014;146:1240–8.
Wong VW, Hui AJ, Wong GL, et al. Four-year outcomes after cessation of tenofovir in immune-tolerant chronic hepatitis B patients. J Clin Gastroenterol. 2018;52:347–52.
Tseng TC, Kao JH. Treating immune-tolerant hepatitis B. J Viral Hepat. 2015;22:77–84.
Acknowledgements
We thank Mr. Dong-Su Jang from the Medical Research Support Section of Yonsei University College of Medicine for designing the figure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Henry Chan has served as an advisory committee member for AbbVie, Aligos, Aptorum, Arbutus, Hepion, Intellia, Janssen, Gilead, Medimmune, Roche, Vaccitech, VenetoRx, Vir Biotechnology and GRAIL; and as a speaker for Mylan, Gilead and Roche. Hye Won Lee declared no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H.W., Chan, H.LY. Unresolved issues of immune tolerance in chronic hepatitis B. J Gastroenterol 55, 383–389 (2020). https://doi.org/10.1007/s00535-020-01665-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01665-z