Abstract
Purpose
Patients with cancer often experience pain that affects their daily activities and quality of life. The analgesic ladder recommended by the World Health Organization has proved insufficient for many, and its scientific basis has been questioned. This retrospective study investigated factors related to adherence to long-term opioid therapy for patients with moderate cancer pain, including an evaluation of low-dose morphine relative to tramadol.
Methods
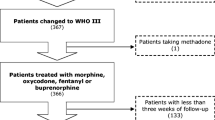
Clinical data were collected of patients with moderate cancer pain (n = 353) who received either low-dose morphine or tramadol and were followed for ≥ 27 weeks. Factors related to regime adherence were investigated, including the analgesia type, cancer therapy (antitumor therapy or palliative care), pain type (nociceptive, neuropathic, or mixed), and living distance to the hospital. Factors related to clinically meaningful pain reduction (≥ 30% reduction in pain from baseline) were also investigated.
Results
Patients taking tramadol, receiving antitumor therapy, experiencing neuropathic pain, and living far from the hospital were more likely to change analgesic strategy compared with, respectively, patients receiving low-dose morphine, palliative care, experiencing nociceptive pain, and living nearby. Factors that increased the likelihood of adherence to the analgesic regime were also associated with the likelihood of clinically meaningful pain reduction. Among adverse effects, a significantly higher percentage of patients experienced constipation in the tramadol group compared with those given morphine.
Conclusions
Among patients with moderate cancer pain, long-term low-dose morphine was safe and more effective than tramadol for clinically meaningful pain reduction, and patients were less likely to change the analgesic strategy.


Similar content being viewed by others
References
WHO Expert Committee (1990) Cancer pain relief and palliative care. World Health Organ Tech Rep Ser 804:1–75
DeAndrea S, Monatanari M, Moja L et al (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19:1985–1991
Mercadante S, Fulfaro F (2005) World Health Organization guidelines for cancer pain: a reappraisal. Ann Oncol 16:iv132–iv135
Ferreira KASL, Kimura M, Teixeira MJ (2006) The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer 14:1086–1093
Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, de Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G, European Palliative Care Research Collaborative (EPCRC), European Association for Palliative Care (EAPC) (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 13:e58–e68
Maltoni M, Scarpi E, Modonesi C, Passardi A, Calpona S, Turriziani A, Speranza R, Tassinari D, Magnani P, Saccani D, Montanari L, Roudnas B, Amadori D, Fabbri L, Nanni O, Raulli P, Poggi B, Fochessati F, Giannunzio D, Barbagallo ML, Minnotti V, Betti M, Giordani S, Piazza E, Scapaticci R, Ferrario S (2005) A validation study of the WHO analgesic ladder: a two-step cf. three-step strategy. Support Care Cancer 13:888–894
Marinangeli F, Ciccozzi A, Leonardis M, Aloisio L, Mazzei A, Paladini A, Porzio G, Marchetti P, Varrassi G (2004) Use of strong opioids in advanced cancer pain: a randomized trial. J Pain Symptom Manag 27:409–416
Sloman R, Wruble AW, Rosen G, Rom M (2006) Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Management Nursing 7(4):153–158
Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpfe A, Petzke F (2014) Long-term opioid use in non-cancer pain. Dtsch Arztebl Int 111(43):732–740
Grond S, Radbruch L, Meuser T, Loick G, Sabatowski R, Lehmann KA (1999) High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manag 18:174–179
Kim HJ, Kim YS, Park SH (2015) Opioid rotation compared with combination for cancer patients with chronic uncontrolled pain: a randomized study. BMC Palliative Care 14(1):1–6
Moksnes K, Kaasa S, Paulsen Ø, Rosland JH, Spigset O, Dale O (2012) Serum concentrations of opioids when comparing two switching strategies to methadone for cancer pain. Eur J Clin Pharmacol 68(8):1147–1156
Santiago-Palma J, Khojainova N, Kornick C, Fischberg DJ, Primavera LH, Payne R, Manfredi P (2001) Intravenous methadone in the management of chronic cancer pain: safe and effective starting doses when substituting methadone for fentanyl. Cancer 92(7):1919–1925
Eisemberg E, Berkey CS, Carr DB et al (1994) Efficacy and safety of nonsteroidal antiinflammatory drugs for cancer pain: a meta-analysis. J Clin Oncol 12:2756–2765
Minotti V, de Angelis V, Rigetti E et al (1998) Double blind evaluation of short-term analgesic efficacy of orally administered diclofenac, diclofenac plus codeine, and diclofenac plus imipramine in chronic cancer pain. Pain 74:133–137
Lowery AE, Greenberg MA, Foster SL, Clark K, Casden DR, Loscalzo M, Bardwell WA (2012) Validation of a needs-based biospychosocial distress instrument for cancer patients. Psychooncology 21:1099–1106
Xuemei L, Huaiqing L, Hengjiang G (2002) Epidural patient control using tramadol compared with morphine for postoperative cancer pain. Mod Med Health 18:122–123
Bandieri E, Romero M, Ripamonti CI, Artioli F, Sichetti D, Fanizza C, Santini D, Cavanna L, Melotti B, Conte PF, Roila F, Cascinu S, Bruera E, Tognoni G, Luppi M, the Early Strong Opioid Treatment Study Investigators (2016) Randomized trial of low-dose morphine compared with weak opioids in moderate cancer pain. J Clin Oncol 34(5):436–442
Seow H, Bainbridge D (2018) A review of the essential components of quality palliative care in the home. J Palliat Med 21(S1):S37–S44
Oh SY, Shin SW, Koh SJ et al (2017) Multicenter, cross-sectional observational study of the impact of neuropathic pain on quality of life in cancer patients. Support Care Cancer 25(12):1–9
Holtzman AL, Williams JP, Hutchinson DF et al (2017) Improving patient-reported pain during radiotherapy through nurse involvement and patient education. J Clin Oncol. https://doi.org/10.1097/COC.0000000000000415
Lampl C, Schweiger C, Haider B, Lechner A (2010) Pregabalin as mono- or add-on therapy for patients with refractory chronic neuropathic pain: a post-marketing prescription-event monitoring study. J Neurol 257(8):1265–1273
Funding
This work was funded by Science & Technology Department of Sichuan Province grant 2012SZ0143.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, Rj., Fu, Y., Zhu, J. et al. Long-term low-dose morphine for patients with moderate cancer pain is predominant factor effecting clinically meaningful pain reduction. Support Care Cancer 26, 4115–4120 (2018). https://doi.org/10.1007/s00520-018-4282-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4282-2