Abstract
Key message
A 6–9 month backward time shift of the carbon uptake gave the highest correlation between annual biomass increment and carbon uptake in this old even aged forest.
Abstract
Plants’ carbon uptake and allocation to different biomass compartments is an important process for both wood production and climate mitigation. Measurements of the net ecosystem carbon dioxide exchange between ecosystems and the atmosphere provide insights into the processes of photosynthesis, respiration and accumulation of carbon over time, and the increase in woody biomass can be assessed by allometric functions based on stem diameter measurements. The fraction of carbon allocated to radial stem growth varies over time, and a lag between carbon uptake and growth can be expected. The dynamics of non-structural carbohydrates and autotrophic and heterotrophic respiration are key mechanisms for understanding this lag effect. In this study, a 9-year record of carbon flux and tree-ring data from Norunda, Sweden was used to investigate the relationship between net and gross carbon uptake and carbon allocated to growth. The flux data were aggregated to monthly sums. When full 12-month periods of accumulated carbon exchange were successively shifted backwards in time, the highest correlation was found with a 6–9 month shift, showing that a large part of the previous growing season was important for explaining the biomass increment of the following year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon uptake by forests has the potential to mitigate climate change, and its allocation to biomass is of great importance for the human society, as the tissues formed are the base for provision of fibre and other goods (EASAC 2017). Even though progress has been made on the understanding of carbon allocation (Cannell and Dewar 1994; Litton et al. 2007), there are still large uncertainties about the allocation to different structural and non-structural compartments. The allocation fractions also vary over shorter and longer time scales in response to environmental variables, and so does the amount that is used for respiration (Brüggemann et al. 2011). The allocation process of greatest interest to forestry is the stem growth, as it is directly linked to the potential harvest (Litton et al. 2007).
Estimates of tree growth are typically based on repeated measurements of tree diameter. These can either be taken outside the bark with calliper, tape-measure or dendrometers, or from tree-ring widths that are measured on an increment core (Metsaranta and Lieffers 2009). From the change in diameter, a change in tree biomass or volume can be estimated after applying allometric relationships, which can be scaled up to stand level if a representative sample of trees has been taken (Dye et al. 2016). This growth of biomass is connected to the ecosystem carbon balance, which means that the net change of carbon in living biomass will be equal to the net uptake of C from the atmosphere if there is a balance between heterotrophic respiration and input of dead material to the soil (Barford et al. 2001).
The eddy covariance (EC) methodology can provide long records of the net ecosystem carbon exchange (NEE) between the ecosystem and the atmosphere with high temporal resolution, typically 30 min (Aubinet et al. 2000). The measured fluxes have been used to study how the processes of photosynthesis and respiration and the resulting NEE are affected by elemental drivers (e.g. Baldocchi 2008). With the increasing number of sites, NEE has been used to describe the spatial variation of the carbon balance for different biomes (Luyssaert et al. 2007). Over time, the processes involved in the NEE will result in an accumulation or loss of carbon called net ecosystem production (NEP = − 1 × NEE), which can be in the soil or biomass (Curtis et al. 2002).
Stem growth normally starts early in spring, often before leaf-out for deciduous trees (Gough et al. 2008), and therefore it depends on stored carbohydrates to a certain degree (Kozlowski 1992). Another aspect is that a large fraction of the new C in the stem structure is incorporated in the cell walls after an initial phase of cell division and enlargement (Cuny et al. 2015). A lag between carbon uptake and growth estimated from radial stem expansion is therefore expected (Kozlowski 1992), but when flux data have been compared to growth estimates, the results are mixed in this respect. For an old-growth black spruce forest in central Manitoba, the 10-year record of growth and NEE data showed a strong relationship between annual growth and NEP but no relationship to gross primary production (GPP) (Rocha et al. 2006). For five European sites, annual wood production estimated from ring widths generally correlated significantly with NEP for the first 6–7 month of the year but the relationships for GPP were weaker (Babst et al. 2014). However, those two studies did not indicate any correlation between growth and NEE data from the previous year. There was, on the other hand, no significant correlation between EC-based and biometrically based NEP on an annual basis, for a hardwood forest in Michigan, but the 5-year accumulated values agreed well (Gough et al. 2008). In the 20-year record from the Howland spruce-fir boreal transition forest, current-year NEP and tree biomass increment were correlated for the second half of years (Teets et al. 2018), but for the first years a significant correlation was found first with a full 12-month shift of NEP data (Richardson et al. 2013). Teets et al. (2018) relate this shift to stressful conditions during spring for a couple of years, but general knowledge about conditions that determines whether carbohydrates are used directly for growth or stored for use in the coming year are lacking.
Seasonal storage is of importance for the trees to handle the asynchrony of supply and demand of carbohydrates, to overcome hazardous situations such as defoliation, and to prepare for seasonal specific demands (Chapin et al. 1990). Moderate drought can result in temporal accumulation of non-structural carbohydrates as photosynthesis is less constrained than growth by such an event (Fatichi et al. 2014; Hartmann and Trumbore 2016). The sink strength for C of different biomass compartments is regulated by seasonal specific demands, whose level often is limited by temperature, nutrients and water (Fatichi et al. 2014). In the beginning of the growing season, roots and stem wood are produced to meet the leaf demand of water and nitrogen, and later in the summer seed production becomes a major sink for carbohydrates (Chapin et al. 1990; Kozlowski 1992). In autumn, carbohydrates are shunted into storage and bud formation, and the winter hardening processes start in connection to this. Carbohydrate reserves will provide resources to support growth and will also protect the trees during winter and spring (Kozlowski 1992) so that in the following year a lower proportion of the GPP will be needed for tree repair and a higher proportion can be used for growth.
In the present study, we use a 9-year record of EC CO2 exchange data from a mixed pine-spruce forest and tree growth estimated as yearly biomass increment (YBI) from tree rings to investigate the relationship between carbon fluxes and growth. The studied site is special in that it is a relatively old forest of mixed species, which due to its management history is a sustained source of CO2 to the atmosphere. Using a monthly time step of the carbon exchange data, the periods of carbon exchange that affect the current-year growth were examined, which address the question whether carbohydrates are used directly for growth or stored for use in the coming year. Our hypothesis is that the carbon exchange during late summer, autumn and early winter is important for the growth next year and information is lost if calendar-year totals of NEP and GPP are used.
Materials and methods
Site description
The studied site, Norunda, is located in Central Sweden (60° 05′ N, 17° 29′ E, 45 m a.s.l.). The ca. 100-year-old forest is a mix of Scots pine (Pinus sylvestris L., 63% of the basal area) and Norway spruce [Picea abies (L.) Karst., 33%] with some deciduous trees dominated by downy birch (Betula pubescens Ehrh.), silver birch (B. pendula Ehrh.) and common alder [Alnus glutinosa (L.) Gaertn.]. In connection with the clear-cut ca. 100 years ago, the area was also ditched, which increased productivity and lowered the water table. The soil is a sandy glacial till with a high fraction of stones and boulders. Bilberry (Vaccinium myrtillus L.) dominates the field layer over most of the area with elements of grasses and ferns. In some wetter depressions, there are Sphagnum mosses and herbs. Mean annual temperature was + 5 °C and precipitation sum 527 mm year−1 in 1961–1990 (data from Uppsala 30 km to the south). More details about the Norunda site can be found in Lundin et al. (1999).
Biomass inventory at stand level 2001
The tree biomass of the whole stand was estimated in 2001 (Håkansson and Körling 2002). Diameter, height, and height to the living crown were measured on all trees on thirteen 625 m2 plots and one 900 m2 plot in a stratified sampling regime within the 300-m radius major footprint area of the flux measurements. In 2001, the tree density was ca. 895 trees ha−1, the basal area was 40.4 m2 ha−1, and the dominant height was 27.5 m. Biomass components (needles, living branches, stem and stump-root system) were calculated for each tree from allometric functions (Marklund 1988), using tree height, height to the living crown and diameter as input. Based on the inventoried area of the strata (As inv), the total area represented by the strata (As) and the total area within the 300-m radius (Atot), tree-level biomass (b) was scaled up to stand-level biomass (B) for each component x in year 2001 based on b of the trees in the different strata (s):
Growth measurements
In December 2002, 15 pines and 15 spruces were sampled for growth determination. Along transects west and southwest from the tower (the dominant wind directions) the diameter of candidate trees were measured. From the candidate trees, samples were taken according to basal area contribution represented in different diameter classes within the 300 m radius (Fig. 1). Two tree cores, 90° apart, were taken at breast height (1.3 m) with a 400-mm-long, 5.15-mm diameter increment borer (Haglöf Sweden AB, Långsele, Sweden). The tree height and diameter at breast height were also measured. The tree-ring widths were measured on an Aniol-measuring machine (Aniol 1987) with a binocular microscope (6–40× magnification) connected to a PC. If the core had missed the pith, the age at breast height was estimated with a “pith locator” (Applequist 1958). Circumferential variation was considered by taking the mean ring width from the two right-angled cores. Crossdating of the tree rings was established using t value (Baillie and Pilcher 1973) and “Gleichläufigkeit” (Eckstein and Bauch 1969) statistical tests.
The basal area within a 300 m radius of the flux tower divided into classes of species and diameter (wide bars, bright shades) and the number of trees sampled for growth estimates from increment cores for the same classes (narrow bars, dark shades). The top left inset shows a histogram of the tree density for the same classes
Tree heights were assigned for each year by assuming that the height development with age followed standard height development curves for different site indexes (H100, i.e. dominant height at the age of 100 years) (Hägglund and Lundmark 1977). For each tree, a specific height curve was determined from the current tree height and number of annual growth rings at breast height (breast height age).
As the allometric functions use diameter outside bark, a bark-thickness function was introduced. Bark thickness was measured on the sample trees on the same occasion and positions as the coring, and to enable extrapolating to younger ages, also on 15 trees of each species from two younger stands in the surroundings. Linear regression established the dependency of bark thickness (T) to diameter and age:
where D and Y are the diameter and age at breast height, respectively, and k1–3 are fitted parameters (Table 1).
The quotient (k4) between the measured bark thickness and that calculated from Eq. (2) in 2002 was used to correct the diameter estimates for single trees. The bark increment from age Y to age Y + 1 can then be expressed as:
D at age Y can be expressed as:
where Winc is the measured wood increment year Y + 1. After substitution with Eq. (3), the relationship simplifies to:
From height and diameter, the biomass components of the cored trees were calculated from allometric functions (Marklund 1988), and then summed up to total of the cored trees (β, kg). The biomass (B, kg m−2) was then calculated for the whole stand for each component x and every year y:
n (stems ha−1) is the stand-level number of trees in year y. The number of trees each year was back-calculated from the current number of trees using a constant self-thinning fraction of 0.2% of the trees that die each year. The biomass from pine and spruce were treated separately and the small fraction of deciduous trees (3% of basal area, Fig. 1) was ignored. The stand-level yearly biomass increment (YBI) for pine, spruce and the total, calculated as By − By−1, was summed for all the tree components and expressed in carbon units using a constant factor of 0.5 g C g−1 dry biomass.
Flux measurements
Net ecosystem exchange (NEE) was measured at a height of 35 m (Grelle and Lindroth 1996). The system used a SOLENT three-dimensional sonic anemometer (Gill Instruments, Lymington, Great Britain) and a LI-6262 infrared gas analyser (LI-COR Inc. Lincoln, Nebraska) with a sampling rate of 10 Hz. The fluxes were calculated based on 30-min averages and were corrected using standard methods (Aubinet et al. 2000; Grelle and Lindroth 1996). The measurements started in spring 1994 and measurements from June 1994 to December 2002 are used in this analysis. Data covered over 90% of the period in each of the years that are considered here.
Regression functions were fitted to 2-month periods of nighttime NEE values:
where R is the average nighttime NEE, Tnight is the average nighttime air temperature, θREW is the relative extractable soil water and a–c are fitted parameters. For periods with missing θREW data or with no dependency of θREW to R, c was set to 1. Volumetric soil water content was measured at 0–20 cm depth and θREW was scaled linearly from 0 to 1 between wilting point (2%) and field capacity (35%). The functions (Eq. 7) were extrapolated to the daytime period using average daytime temperature, and the gross primary production (GPP) was calculated as daytime R minus daytime NEE. Gaps in NEE up to 2 h (0.27% of the total amount of data) were replaced through linear interpolation. For longer gaps (3.7% of the total amount of data), GPP was modelled with a light-response function:
where ε is the initial slope of the light response, Asat the saturated GPP level and Ig incoming global radiation. The function was fitted daily from a 10-day moving window of data, and NEE was calculated from modelled R and GPP. Net ecosystem production was calculated as − 1 × NEE. NEP and GPP were then summed for 1-month periods for further analysis. Flux measurements also continued after the sampling of tree-ring data, but due to some technical problems during the following 2 years along with changes in instrumentation and a later thinning activity, this extended time series was not included in the present study.
Data analysis
To identify significant differences in YBI between years, a pairwise comparison of YBI for single trees with a two-way ANOVA (tree × year) and Bonferroni correction was applied in SPSS (SPSS Inc., Chicago, IL, USA). The analysis was done for all sampled trees, as well as for pine and spruce separately.
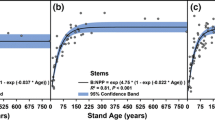
There was a trend of decreasing YBI with time (Fig. 2c). One reason for such a decrease could be a diminishing effect of a fertilization treatment about 30 years prior to the study. Another reason can be an age-related decline, due to increased maintenance respiration and increased reproductive effort when the stand gets older (Ryan et al. 1997). As this trend is more related to the partitioning of GPP than to the uptake, it motivated removal of this trend before the comparisons to NEP and GPP. Results are presented with and without elimination of this trend in growth.
Time series of yearly GPP (a), NEP (b), pine and spruce total biomass increment (c), with mean values shown to the right. GPP and NEP were also calculated with a 7-month timeshift (i.e. from June the previous year to May the growth year) (d, e). The regression lines in c have the following statistics: pine growth r2 = 0.09, P = 0.42; spruce growth r2 = 0.29, P = 0.13
To assess the period of accumulated NEP or GPP on which the YBI depended, linear regressions were performed between YBI and GPP or NEP calculated for all possible periods starting from April of the year before the YBI value to October of the year of the YBI value with a 1-month time resolution. Residuals were checked for normality using a Shapiro–Wilk test (Royston 1995).
Data availability
The datasets generated and used during the current study are available from the corresponding author on reasonable request.
Results
Trends in NEP, GPP and growth
The annual GPP averaged 957 g C m−2 with a minimum of 851 and a maximum of 1058 g C m−2 (Fig. 2a). For NEP the mean was − 52 g C m−2 year−1, which varied between − 105 and + 15 g C m−2 (Fig. 2b). The tree carbon accumulation estimated from annual ring width at breast height and allometric functions (YBI) was on average 190 g C m−2 year−1 (range 165–224 g C m−2) of which 113 g C m−2 year−1 was for pine (101–138 g C m−2) and 77 g C m−2 year−1 for spruce (64–99 g C m−2) (Fig. 2c). The stem part of the increase was 137 g C m−2 year−1 (data not shown). Using the biomass expansion factors of Lehtonen et al. (2004), this corresponds to a growth of 6.8 m3 ha−1 year−1, which is close to the average (7.0 m3 ha−1 year−1) for the county of Uppland (Nilsson and Cory 2018). Assuming a constant amount of field vegetation C and no export by herbivory or dissolved organic carbon, the difference between NEP and YBI must be attributed to a change in soil carbon, which would imply that the soil in Norunda on average lost 242 g C m−2 year−1 (NEP − YBI). The difference in YBI between years was only significant for the years with most extreme growth (see Online Resource Table S1).
Relationships between growth and NEP or GPP
There was no relationship at all between YBI and calendar-year NEP or GPP (Fig. 3). When full 12-month periods successively were shifted backwards, maximum positive correlation was found with a 6–9 months’ shift depending on species, whether YBI was detrended or not and if NEP or GPP was assessed (Fig. 4). The optimal shift was about a month longer for NEP than GPP. A 7-month shift implies that the current-year growth was related to the carbon exchange for the period from previous-year June to current-year May. The maximum r2 was higher for pine and total YBI and lower for spruce. The use of detrended YBI clearly improved the correlation for GPP but not for NEP.
Coefficients of determination (r2) for the correlation between biomass increment and NEP or GPP. The yearly total NEP and GPP data were summed for 12-month periods shifted 0–9 months backwards. For example, the time shift of 7 months means that the calendar-year growth is paired to NEP or GPP for the period from previous-year June to May the current year. In b and d the trend in the growth data (see Fig. 2c) has been eliminated. Negative r2 values means that the correlation was negative. For r2 > 0.50 the P value is < 0.05
The robustness of the results was tested by sequentially removing one of the years in the regressions. Though r2 varied with removed year, the optimal time shift of 7–9 months for the NEP data was confirmed (Online Resource Fig. S1). For GPP, removing year 1997 from the regressions for pine and the total also gave a high r2 for a short time shift of 0–4 months, but except for that case, the typical result of highest correlation with a longer time shift was repeated with all other combinations of excluded year (Online Resource Fig. S2).
When all possible time periods over the last 10 months were considered, it was clear that the carbon uptake from the previous year’s growing season could explain a lot of the variation in YBI (Fig. 5, Online Resource Table S2). Starting the accumulation in June or July of the previous year was best for GPP while starting earlier in April or May was better for NEP. A period of accumulated NEP or GPP data in the beginning of the current year ending in April or May also explained a lot of the variation in YBI for both species, despite low uptake during this period (Fig. 5, Online Resource Table S2). The Shapiro–Wilk test indicated normality of the residuals in most cases (220 of 230 cases with P < 0.05 for the regressions). The cases of significant non-normality were mostly present in the non-detrended YBI data (9 of 10 cases with P < 0.05 for the S–W test).
Relationship between yearly stand level biomass increment (YBI), for pine, spruce and the total, and accumulated NEP or GPP for all possible time periods (start month at y-axis, end month at x-axis) from April of the year previous to the YBI value to October of the growth year. Along the diagonal the period is 1 month, increasing to 19 months in the bottom right. Shaded abbreviations means fluxes from months the year before the YBI value. Negative r2 values means that the correlation was negative. Significant (P < 0.05) positive correlations are shown with a dot
The between-year variation in carbon exchange (Fig. 6) shows that the variation in GPP can be mainly attributed to the summer months while for NEP the variation is also large for October–December. The year with the highest YBI (1997) was preceded by unusually high NEP and GPP the previous summer, and in 2000, a year with high pine growth, GPP was high in June of the previous year (Fig. 7).
Deviation from the monthly means of NEP and GPP for selected periods. The straight lines represent average monthly deviation of NEP or GPP for the period from June the year before to May, which was the 12-month period with generally the best correlation to growth (Fig. 4). The years presented were selected for having high (1997 and 2000) or low (1998 and 2001) detrended growth compared to the average (Fig. 2c)
Discussion
In this study, neither NEP nor GPP summed over a calendar-year 12-month period was correlated to growth (Fig. 3). A time shift of about 2 months for the accumulation of annual NEP has been suggested for temperate forests to better match the rhythm of the forest (Peichl et al. 2010; Urbanski et al. 2007), but a 2-month shift was too short to improve the relationship in the present study (Fig. 4). Zweifel et al. (2010) suggested a dynamic start of the NEP year with a time shift corresponding to the date in the growing season when NEP turns negative. Because heterotrophic respiration (Rh) is so high in Norunda that date will already be reached in July or August (Fig. 6), suggesting a time shift of 5–6 months, which is more in line with the end of stem growth measured in an adjacent stand (Lagergren and Lindroth 2004) (Online Resource Fig. S3) and the time shift of 6–9 months with the highest correlation to growth (Fig. 4). For a boreal pine forest in Hyytiälä, April was the single month with the best correlation to growth for both NEP and GPP (Babst et al. 2014), and also in our study the carbon uptake in the first 4–5 month of the year had a high correlation. However, including NEP or GPP from the previous year did not improve the relationships in the study of Babst et al. (2014). Nonetheless, we found that including data from the year before substantially improved correlation for both NEP and GPP (Fig. 5, Online Resource Table S2). Lag effects discussed below may explain the mismatch between carbon uptake and growth.
On linking NEP and GPP to growth
A mismatch between carbon fluxes and growth is predictable because carbon uptake and change in stem width are not directly related for several reasons: (1) the carbon is stored for some time in the tree before it is allocated to tissues in the stem or other biomass compartments (Kozlowski 1992; Richardson et al. 2013), and tree growth is often not limited by the supply of C (Körner 2003). According to the pipe theory (Shinozaki et al. 1964a, b), the primary purpose of new xylem formation in the stem is to support water transport to the needles for transpiration. As pine and spruce have their shoot growth pre-determined from previous year’s bud formation (Kozlowski 1964), the trees will allocate their carbon to other sinks such as roots and storage, after elongating the shoots and producing new xylem that is wide enough to provide required water-transport capacity to the new needles. (2) The allocation between stem and other tissues varies over time (Bouriaud et al. 2005; Litton et al. 2007). Annual tree height measurement would have decreased some of this uncertainty but for trees around 100 years old, the height increase is small and has a minor influence on estimates of biomass increase compared to the change in diameter. (3) The cell division in new wood cells, the size increase of the new cells, and cell wall thickening are three separate processes that cause a lag between circumference increase and woody biomass production of about a month in boreal forests (Cuny et al. 2015). (4) The wood density can vary between years (Bouriaud et al. 2004), though the effect of correcting for variable density was < 5% on annual wood increment for four out of five European forests (Babst et al. 2014). We used a constant density of 0.5 g C g−1 dry biomass, which adds an uncertainty to the YBI estimates. (5) The fraction of GPP that goes to autotrophic respiration (Ra) varies with environmental variables (Braswell et al. 2005).
Another cause of the mismatch is that there are other ecosystem components that disrupt a direct comparison between growth and NEP or GPP. In theory, NEP = YBI + YBD + H − Rh + E, where YBD is the production of detritus from dead trees and turnover of needles, branches, roots and ground vegetation, and H is herbivory [often treated as a negligible term in healthy forest (Schowalter et al. 1986)]. E is the mismatch of estimated YBI from measured change in diameter and actual change in biomass, caused by the factors described above, measurement errors, bias in the allometric functions, representativeness of the tree-ring sample and the footprint of the EC flux and scaling up tree growth to stand level. If there is a balance in the soil between YBD input and Rh loss, NEP should equal YBI (e.g. as found by Ohtsuka et al. 2009), but the soil in Norunda loses on average 242 g C m−2 year−1 according to the balance of the mean values in Fig. 2. For GPP, the components can be expressed as, GPP = YBI + YBD + H + Ra + E. Both comparisons of NEP and GPP with growth are therefore disrupted by YBD and a respiratory term. The components of YBD are not easy to measure and YBD/YBI estimates vary widely among studies, e.g. from 61% by Granier et al. (2008) to 205% by Gough et al. (2008). While Ra is directly influenced by temperature and growth (Ryan 1991), Rh is more complicated to measure as soil moisture and input of YBD, that to some extent varies randomly with events of mortality, also affect the flux level (Davidson et al. 2006). In this study, we assume that the fractions among components were relatively stable over years, as no major disturbances occurred, but the high level of Rh makes the comparison between NEP and YBI less direct. The correlation between YBI and growth was, however, generally higher for NEP than for GPP, as also found at other sites (Babst et al. 2014).
There is also unknown bias in the EC data due to: variable footprint, possible advection, low mixing conditions at night, gap filling of NEE and for GPP also partitioning of NEE (Oren et al. 2006; Papale et al. 2006). Generally the uncertainty of NEP and GPP for these factors is about 10–30% for annual sums (Papale et al. 2006). In the present study, we implemented a conservative method for partitioning NEE, using average nighttime NEE and 2-month periods for fitting the temperature dependency, which resulted in about 10% lower annual GPP compared to a method using 30-min data and half-month periods (Lagergren et al. 2008). The conservative method was used to avoid uncertain exponential extrapolation of often poorly fitted nighttime data. Data processing can also introduce errors in the scaling of tree-ring samples to stand-level growth. In the present study, the number of sampled trees was proportional to the BA of different diameter classes, but each sampled tree covers a smaller fraction of the total BA of the smaller classes (Fig. 1). This means that the scaling results, using the proportional change of the summed growth of the sampled trees (Eq. 6), could have been biased if the relative change in growth was dependent on diameter. Such a dependency was, however, not found (Online Resource Fig. S4a), and also a dependency on diameter for the trend with time (Fig. 2c) was absent (Online Resource Fig. S4b). There can also be bias in the allometric functions, but the relative change in biomass compartments for a change in diameter is quite insensitive to such errors (Ståhl et al. 2014).
Direct effects
Seasonal estimates of accumulated GPP and growth indicate that stem growth depends on stored carbohydrates as the maximum rate of diameter-based increase in biomass exceeds net primary production (NPP) from flux measurements (Online Resource Fig. S3). The increase in circumference is almost completed at the end of July while about 1/3 of yearly GPP takes place after that date, but the carbon allocation to the stem lags behind the increase in diameter (Cuny et al. 2015). Based on empirical data, Richardson et al. (2013) showed that modelling stem growth with two pools (fast and slow) of stored carbon greatly improved model performance of inter-annual variation of growth. For spruce, NEP from the previous summer (June–August) explained more than 50% of the variation in growth (Online Resource Table 2a, 2c). The carbohydrate pool of Norway spruce has been found to differ more than 20% between different years during autumn (Flower-Ellis 1993).
A clear signal of a time shift between net or gross CO2 uptake and growth may only be detectable when there are marked differences among the growing seasons, and since extreme events are relatively rare, this signal may go undetected if short time series are used. The most extreme months deviated about 60 g C m−2 for GPP and 35 g C m−2 for NEP from the mean (Figs. 6, 7), which is 81% and 70% of the standard deviation of the annual sums (Fig. 2a, b), respectively. For an old-growth black spruce site, there was a high correlation between YBI and NEP but no correlation to GPP (Rocha et al. 2006). There was, however, a strong trend in both NEP and growth over time in that study, so it is likely that some other driver was responsible for the trend in YBI. Rocha et al. (2006) tried to lag NEP and GPP in full-year steps but it decreased the correlation for NEP and did not lead to the detection of any correlation for GPP. However, Gough et al. (2008) found that biometrical estimates of NPP were correlated with EC-based NPP from the previous year but not current, which is more in line with our results.
Indirect effects
The year 1997 had poor GPP in the summer (Fig. 7), which was followed by low YBI in 1998, especially for spruce. Lagergren et al. (2008) showed that the photosynthetic capacity in the summer is a strong driver for between-year variation of annual GPP in Norunda and other Nordic forests. The low GPP in 1997 can indeed be explained with a low photosynthetic capacity, but the reason for that could not be supported by exceptional weather (Lagergren et al. 2008). Rather, it could have been caused by some overlooked environmental or biotic factor. A summer–autumn with low GPP can be an effect of drought (Ciais et al. 2005), insects (Allard et al. 2008) or fungi (Wang et al. 2017). As this will lead to loss of needles, a higher proportion of the GPP next year will be used for needles and it could also trigger a higher reproductive effort (Kozlowski 1992), which means that a lower proportion of GPP will go to stem growth. Reduced needle area the previous year could also promote reduced need for xylem growth next year according to the pipe theory described above, as conifers with several age classes of needles cannot replace a big loss of leaf area in 1 year (Kozlowski 1992; Schowalter et al. 1986).
In a warm and wet autumn and early winter, both Rh and Ra will be high and consequently NEP will be low. The high Ra will reduce the stored C in the trees and could lead to reduced winter hardiness (Ögren et al. 1997). Next spring a higher proportion of GPP and storage have to be used to repair the photosynthetic capacity that has been damaged during the winter. Year 2000 was such a year with high losses of carbon October–December because of very warm (mean air temperature 5.2 °C compared to 1.5 °C average for 1994–2003) and wet (289 mm precipitation compared to 154 mm) conditions that were followed by poor growth of both pine and spruce in 2001 (Fig. 7).
Conclusions
This study showed a time shift between net and gross CO2 uptake and growth, as part of the previous growing season explained a portion of the variation in the biomass increment of the following year. The dynamical storage of non-structural carbohydrates is a key mechanism for understanding this lag effect, but weather-induced variation in autotrophic and heterotrophic respiration and variable allocation to different tissues are also important. Based on selected years with higher or lower growth than average, we have provided some explanations why NEP and/or GPP, for periods in the previous year, can affect growth.
Author contribution statement
FL performed the increment coring and most of data analysis and writing. AMJ took part in data analysis and writing. HL took part in the design of the tree sampling, performed the tree ring analysis and have commented on the manuscript. AL initiated the study, was PI for the eddy covariance measurements and have commented on the data analysis and manuscript.
References
Allard V, Ourcival JM, Rambal S, Joffre R, Rocheteau A (2008) Seasonal and annual variation of carbon exchange in an evergreen Mediterranean forest in southern France. Glob Change Biol 14:714–725
Aniol RW (1987) A new device for computer assisted measurements of tree-ring widths. Dendrochronologia 5:135–141
Applequist MB (1958) A simple pith locator for use with off-center increment cores. J For 56:141
Aubinet M, Grelle A, Ibrom A et al (2000) Estimates of the annual net carbon and water exchange of forests: the EUROFLUX methodology. Adv Ecol Res 30:113–175
Babst F, Bouriaud O, Papale D et al (2014) Above-ground woody carbon sequestration measured from tree rings is coherent with net ecosystem productivity at five eddy-covariance sites. New Phytol 201:1289–1303
Baillie MGL, Pilcher J (1973) A simple crossdating program for tree-ring research. Tree-Ring Bull 33:7–14
Baldocchi D (2008) Breathing of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust J Bot 56:1–26
Barford CC, Wofsy SC, Goulden ML, Munger JW, Pyle EH, Urbanski SP, Hutyra L, Saleska SR, Fitzjarrald D, Moore K (2001) Factors controlling long- and short-term sequestration of atmospheric CO2 in a mid-latitude forest. Science 294:1688–1691
Bouriaud O, Bréda N, Le Moguédec G, Nepveu G (2004) Modelling variability of wood density in beech as affected by ring age, radial growth and climate. Trees 18:264–276
Bouriaud O, Bréda N, Dupouey J-L, Granier A (2005) Is ring width a reliable proxy for stem-biomass increment? A case study in European beech. Can J For Res 35:2920–2933
Braswell BH, Sacks WJ, Linder E, Schimel DS (2005) Esimating diurnal to annual ecosystem parameters by synthesis of a carbon flux model with eddy covariance net ecosystem exchange observations. Glob Change Biol 11:335–355
Brüggemann N, Gessler A, Kayler Z et al (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489
Cannell MGR, Dewar RC (1994) Carbon allocation in trees—a review of concepts for modelling. Adv Ecol Res 25:59–104
Chapin FS, Schulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Ciais P, Reichstein M, Viovy N et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Cuny HE, Rathgeber CBK, Frank D et al (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat Plants 1:15160 (1–6)
Curtis PS, Hanson PJ, Bolstad P, Barford C, Randolph JC, Schmid HP, Wilson KB (2002) Biometric and eddy-covariance based estimates of annual carbon storage in five eastern North American deciduous forests. Agric For Meteorol 113:3–19
Davidson EA, Janssens I, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob Change Biol 12:154–164
Dye A, Plotkin AB, Bishop D, Pederson N, Poulter B, Hessl A (2016) Comparing tree-ring and permanent plot estimates of aboveground net primary production in three eastern US forests. Ecosphere 7:e01454 (1–13)
EASAC (2017) Multi-functionality and Sustainability in the European Union’s Forests. EASAC policy report 32. European Academies Science Advisory Council
Eckstein D, Bauch J (1969) Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwissenschaftliches Centralblatt 88:230–250
Fatichi S, Leuzinger S, Körner C (2014) Moving beyond photosynthesis: from carbon source to sink-driven vegetation modeling. New Phytol 201:1086–1095
Flower-Ellis JGK (1993) Dry-matter allocation in Norway spruce branches: a demographic approach. Stud For Suec 191:51–73
Gough CM, Vogel CS, Schmid HP, Su H-B, Curtis PS (2008) Multi-year convergence of biometric and meteorological estimates of forest carbon storage. Agric For Meteorol 148:158–170
Granier A, Bréda N, Longdoz B, Gross P, Ngao J (2008) Ten years of fluxes and stand growth in a young beech forest at Hesse, North-eastern France. Ann For Sci 65:704
Grelle A, Lindroth A (1996) Eddy-correlation system for long-term monitoring of fluxes of heat, water vapour and CO2. Glob Change Biol 2:297–307
Hägglund B, Lundmark J-E (1977) Site index estimation by means of site properties: Scots pine and Norway spruce in Sweden. Stud For Suec 138:1–38
Håkansson J, Körling A (2002) Uppskattning av mängden kol i trädform-en metodstudie. Master thesis 86, Department of Physical Geography, Lund University
Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees—from what we can measure to what we want to know. New Phytol 211:386–403
Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17
Kozlowski TT (1964) Shoot growth in woody plants. Bot Rev 30:335–392
Kozlowski TT (1992) Carbohydrate sources and sinks in woody plants. Bot Rev 58:107–222
Lagergren F, Lindroth A (2004) Variation in sapflow and stem growth in relation to tree size, competition and thinning in a mixed forest of pine and spruce in Sweden. For Ecol Manag 188:51–63
Lagergren F, Lindroth A, Dellwik E, Ibrom A, Lankreijer H, Launiainen S, Mölder M, Kolari P, Pilegaard K, Vesala T (2008) Biophysical controls on CO2 fluxes of three Northern forest based on long-term eddy covariance data. Tellus B 60:143–152
Lehtonen A, Mäkipää R, Heikkinen J, Sievänen R, Liski J (2004) Biomass expansion factors (BEFs) for Scots pine, Norway spruce and birch according to stand age for boreal forests. For Ecol Manag 188:211–224
Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Glob Change Biol 13:2089–2109
Lundin L-C, Halldin S, Lindroth A et al (1999) Continuous long-term measurements of soil-plant-atmosphere variables at a forest site. Agric For Meteorol 98–99:53–73
Luyssaert S, Inglima I, Jung M et al (2007) CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Change Biol 13:2509–2537
Marklund LG (1988) Biomassafunktioner för tall, gran och björk i Sverige (Biomass functions for pine, spruce and birch in Sweden). Report 45. Department of Forest Survey, Swedish University of Agricultural Sciences, Umeå
Metsaranta JM, Lieffers VJ (2009) Using dendrochronology to obtain annual data for modelling stand development: a supplement to permanent sample plots. Forestry 82:163–173
Nilsson P, Cory N (2018) Forestry statistics 2013—official statistics of Sweden. Infra Service, SLU, Umeå
Ögren E, Nilsson T, Sundblad LG (1997) Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over-wintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant Cell Environ 20:247–253
Ohtsuka T, Saigusa N, Koizumi H (2009) On linking multiyear biometric measurements of tree growth with eddy covariance-based net ecosystem production. Glob Change Biol 15:1015–1024
Oren R, Hsieh C-I, Stoy P, Albertson J, McCarthy HR, Harrel P, Katul GG (2006) Estimating the uncertainty in annual net ecosystem carbon exchange: spatial variation in turbulent fluxes and sampling errors in eddy-covariance measurements. Glob Change Biol 12:883–896
Papale D, Reichstein M, Aubinet M et al (2006) Towards a standardized processing of net ecosystem exchange measured with eddy covariance technique: algorithms and uncertainty estimation. Biogeosciences 3:571–583
Peichl M, Brodeur JJ, Khomik M, Arain MA (2010) Biometric and eddy-covariance based estimates of carbon fluxes in an age-sequence of temperate pine forests. Agric For Meteorol 150:952–965
Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu XM (2013) Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol 197:850–861
Rocha AV, Goulden ML, Dunn AL, Wofsy SC (2006) On linking interannual tree ring variability with observations of whole-forest CO2 flux. Glob Change Biol 12:1378–1389
Royston P (1995) Remark AS R94: a remark on algorithm AS-181: the W-test for normality. J R Stat Soc C Appl 44:547–551
Ryan MG (1991) Effects of climate change on plant respiration. Ecol Appl 1:157–167
Ryan MG, Binkley D, Fownes JH (1997) Age-related decline in forest productivity: pattern and process. Adv Ecol Res 27:213–262
Schowalter TD, Hargrove WW, Crossley DA (1986) Herbivory in forested ecosystems. Annu Rev Entomol 31:177–196
Shinozaki K, Yoda K, Hozuma K, Kira T (1964a) A quantitative theory of plant form—the pipe model theory. I. Basic analyses. Jpn J Ecol 14:97–105
Shinozaki K, Yoda K, Hozuma K, Kira T (1964b) A quantitative theory of plant form—the pipe model theory. II. Further evidence of the theory and its application in forest ecology. Jpn J Ecol 14:133–139
Ståhl G, Heikkinen J, Petersson H, La JR, Holm S (2014) Sample-based estimation of greenhouse gas emissions from forests—a new approach to account for both sampling and model errors. For Sci 60:3–13
Teets A, Fraver S, Hollinger DY, Weiskittel AR, Seymour RS, Richardson AD (2018) Linking annual tree growth with eddy-flux measures of net ecosystem productivity across twenty years of observation in a mixed conifer forest. Agric For Meteorol 249:479–487
Urbanski S, Barford C, Wofsy S, Kucharik C, Pyle E, Budney J, McKain K, Fitzjarrald D, Czikowsky M, Munger JW (2007) Factors controlling CO2 exchange on timescales from hourly to decadal at Harvard forest. J Geophys Res 112:G02020. https://doi.org/10.1029/2006JG000293
Wang X, Stenström E, Boberg J, Ols C, Drobyshev I (2017) Outbreaks of Gremmeniella abietina cause considerable decline in stem growth of surviving Scots pine trees. Dendrochronologia 44:39–47
Zweifel R, Eugster W, Etzold S, Dobbertin M, Buchmann N, Häsler R (2010) Link between continuous stem radius changes and net ecosystem productivity of a subalpine Norway spruce forest in the Swiss Alps. New Phytol 187:819–830
Acknowledgements
The study was founded by the European Commission (project Carbo-Age, Contract no. EVK2-CT-1999-00045), and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning–FORMAS (project “Climate change impact on tree defence capacity”, 2010-822). The study has been conducted under BECC (Biodiversity and Ecosystem Services in a Changing Climate) and LUCCI (Lund University Centre for studies of Carbon Cycle and Climate Interactions), two strategic research areas of Lund University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Leavitt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lagergren, F., Jönsson, A.M., Linderson, H. et al. Time shift between net and gross CO2 uptake and growth derived from tree rings in pine and spruce. Trees 33, 765–776 (2019). https://doi.org/10.1007/s00468-019-01814-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-019-01814-9