Abstract.
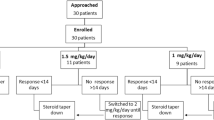
A retrospective cohort study was conducted by the Southwest Pediatric Nephrology Study Group (SPNSG) to address whether a longer initial course of corticosteroids in patients with idiopathic nephrotic syndrome (INS) provides superior protection against relapse without increased adverse effects. In order to be included in the evaluation, patients with INS must have responded to an initial steroid course, either standard or long regimen as defined here, and completed at least 1 year of follow-up. The standard regimen consisted of prednisone 2.0±0.3 mg/kg per day or 60±10 mg/m2 per day for 28±4 days, followed by alternate-day prednisone for 4–12 weeks. The long regimen consisted of daily prednisone 2.0±0.3 mg/kg per day or 60±10 mg/m2 per day for 42±6 days, followed by alternate-day prednisone for 6–14 weeks. The primary outcome measure was relapse of NS within 12 months of discontinuing the initial course of prednisone. There were 151 children who met the criteria for the study; 82 received the standard regimen and 69 the long regimen. The two groups did not differ in age, race, blood pressure, serum albumin, or serum cholesterol prior to the initial steroid course. The cumulative prednisone dose was 49% higher in the long regimen group than in the standard regimen group. Relapse within 12 months was reported in 72.5% of patients who received the long regimen versus 84.1% of those who received the standard regimen. The odds ratio for relapse within 12 months was 0.496 (95% confidence interval 0.22, 1.088), long versus standard regimen. This did not reach statistical significance (χ 2=3.058, P=0.08). The odds ratio of experiencing at least one side effect was 3.76, long relative to standard regimen (n=133, P<0.001). Our data suggest that prolongation of the steroid treatment for the initial episode of steroid-sensitive NS may have a beneficial effect, but at the cost of increased side effects. However, definitive conclusions are limited by the retrospective design of the study and the number of patients. This may have caused failure to achieve statistical significance on the basis of a type II error.
Similar content being viewed by others
References
Barnet HL (1975) The natural and treatment history of glomerular diseases in children. What can we learn from international cooperative studies? Proceedings of the 6th International Congress of Nephrology. Karger, Basel, pp 470–485
Lande MB, Leonard MB (2000) Variability among pediatric nephrologists in the initial therapy of nephrotic syndrome. Pediatr Nephrol 14:766–769
Evans JHC, Long E (1998) A national audit of nephrotic syndrome: the initial course of prednisone and outcome. Pediatr Nephrol 12:C154
Ueda N, Chihara M, Kawaguchi S, Niionomi Y, Nonoda T, Matsumoto J, Ohnishi M, Yasaki T (1988) Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr 112:122–126
Ehrich JHH, Brodehl J Arbeitsgemeinschaft für Pädiatrische Nephrologie (1993) Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Eur J Pediatr 152:357–361
Ksiażek J, Wyszyńska T (1995) Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 84:889–893
Norero C, Rosati P, Lagos E, Delucchi A (1996) Initial treatment of primary nephrotic syndrome in children. Chilean Cooperative Group for Study of Nephrotic Syndrome in Children. Rev Med Chil 124:567–572
Bagga A, Hari P, Srivastava RN (1999) Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 13:824–827
Hodson EM, Knight JF, Willis NS, Craig JC (2000) Corticosteroid therapy in nephrotic syndrome: a meta-analysis of randomised controlled trials. Arch Dis Child 83:45–51
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
British Association for Paediatric Nephrology and Research Unit, Royal College of Physicians (1994) Consensus statement on management and audit potential for steroid responsive nephrotic syndrome. Arch Dis Child 70:151–157
Niaudet P, Broyer M (2000) Management of steroid-responsive nephrotic syndrome. Pediatr Nephrol 14:770–771
Author information
Authors and Affiliations
Additional information
Centers and investigators participating in this study:
Hospital for Sick Children, Toronto, Canada (Gerald Arbus, MD)
Schneider Children's Hospital, New Hyde Park, New York (Bernard Gauthier, MD)
University of Texas HSC, Houston, Texas (Melvin Bonilla-Felix, MD; current address: University of Puerto Rico, Pediatric Nephrology, Science Campus, PO Box 365067, San Juan, PR 00936)
St. Christopher's Hospital for Children, Philadelphia, Pennsylvania (Jorge Baluarte, MD; currrent address: Children's Hospital of Philadelphia, Pediatric Nephrology, 34th and Civic Center Boulevard, Philadelphia, PA 1910, USA)
Babies and Children's Hospital, New York, New York (Martin Nash, MD)
North Texas Hospital for Children, Dallas, Texas (Ronald Hogg, MD, Jennifer Gray, RN)
University of Tennessee, Memphis, Tennessee (Shane Roy, III, MD)
Children's Hospital Medical Center, Cincinnati, Ohio (C. Frederic Strife, MD)
Children's Hospital of Philadelphia, Philadelphia, Pennsylvania (Mary Leonard, MD)
University of Rochester, Rochester, New York (Melissa Gregory, MD; current address: University of Michigan Medical Center, Mott F6865, 1505 Simpson Road, E., Ann Arbor, MI 48109, USA)
Oregon Health Sciences University, Portland, Oregon (Marc Lande, MD; currrent address: University of Rochester Medical Center, Pediatric Nephrology, 601 Elmwood Avenue, Box 777, Rochester, NY 14642, USA)
University of California at Los Angeles, Los Angeles, California (Ora Yadin, MD)
University of Texas Medical Branch, Galveston, Texas (Steven Diven, MD)
Rights and permissions
About this article
Cite this article
Lande, M.B., Gullion, C., Hogg, R.J. et al. Long versus standard initial steroid therapy for children with the nephrotic syndrome. Pediatr Nephrol 18, 342–346 (2003). https://doi.org/10.1007/s00467-002-1052-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-002-1052-6