Abstract
The Eating Assessment Tool-10 (EAT-10) is used worldwide to screen people quickly and easily at high risk for swallowing disorders. However, the best EAT-10 cutoff value is still controversial. In this systematic review and meta-analysis, we estimated and compared the diagnostic accuracy of EAT-10 cutoff values of 2 and 3 for screening dysphagia. We searched the PubMed, Web of Science, EMBASE, Cochrane Library, CNKI, WANFANG, and VIP databases from May 2008 to March 2022. The meta-analysis included 7 studies involving 1064 subjects from 7 different countries. Two studies were classified as high quality and five studies as medium quality. With an EAT-10 cutoff value of 2, using flexible endoscopic evaluation of swallowing or video fluoroscopic swallowing study as the gold standard, the pooled sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratio were 0.89 (95% confidence interval [CI] 0.82–0.93), 0.59 (95% CI 0.39–0.77), 2.17 (95% CI 1.38–3.42), 0.19 (95% CI 0.13–0.29), and 11.49 (95% CI 5.86–22.53), respectively. When a cutoff of 3 was used, these values were 0.85 (95% CI 0.68–0.94), 0.82 (95% CI 0.65–0.92), 4.84 (95% CI 1.72–13.50), 0.18 (95% CI 0.07–0.46), and 26.24 (95% CI 5.06–135.95), respectively. Using EAT-10 cutoff values of 2 and 3, the areas under the curve were 0.873 (95% CI 0.82–0.93) and 0.903 (95% CI 0.88–0.93), respectively, showing good diagnostic performance. EAT-10 can be used as a preliminary screening tool for dysphagia. However, a cutoff of 3 is recommended for EAT-10 due to better diagnostic accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia refers to a sensation or sign of an underlying health problem in which food or liquid has difficulty entering the stomach [1,2,3]. Aging is an independent risk factor for swallowing disorders, and the prevalence of dysphagia among adults over 60 years of age living independently is 11.4–33.7% [4, 5]. Addition, cerebrovascular and neurodegenerative diseases cause a broad spectrum of neurological disorders and lead to difficulty in swallowing [6]. The prevalence of dysphagia in stroke is 29–64% [7, 8], 50–75% in Alzheimer’s disease [9], and can be as high as 82% in Parkinson’s [10]. Head and neck cancer, trauma, gastroesophageal reflux disease, and primary esophageal abnormalities are also common causes of dysphagia [11]. The health and economic impact of dysphagia on patients are enormous, and common complications include dehydration, malnutrition, and aspiration pneumonia. Patients with dysphagia have significantly longer hospital stays, are four times more likely to be readmitted within a month, and have a 13-fold higher mortality rate during hospitalization [12,13,14]. Bonilha et al. [15] followed patients with dysphagia for 1 year after controlling for influencing factors and found that annual medical costs for these patients were 4510 USD higher than for patients without dysphagia. At present, the video fluoroscopic swallowing study (VFSS) and flexible endoscopic evaluation of swallowing (FEES) are considered the gold standards for the diagnosis of dysphagia, both of which are imaging methods with high consistency in penetration, aspiration, and pharyngeal residue assessments [16, 17]. Eleven studies [18,19,20,21,22,23,24,25,26,27] have reported that patients are exposed to x-ray radiation for 2.5 to 18 min during VFSS examination, and Bonilha et al. [28] assessed 612 patients and identified an average exposure time of 2.9 min. FEES is only for local observation, and it cannot be used to observe pharyngeal contraction, laryngeal activity, or cricopharyngeal muscle opening during swallowing [29]. At the same time, owing to individual economic and medical development levels and health policies, it is difficult to evaluate every patient at risk of swallowing disorders. These individuals should first be screened using a questionnaire and those with suspected dysphagia can be further evaluated using imaging evaluation. Early screening can identify people at risk for swallowing disorders, and appropriate feeding strategies and treatment can also improve patient prognosis and reduce the incidence of aspiration pneumonia [30]. Although several screening tools for dysphagia have been developed, few are actually widely used in clinical practice. Sensitivity and specificity are important indicators of the performance of screening tools, but some screening tools have low diagnostic accuracy. There are little data on sensitivity and specificity for the Sydney Swallow Questionnaire (SSQ), the Dysphagia Risk Assessment for Community-Dwelling Older Adults (DRACE), and the Ohkuma questionnaires [31, 32]; the Modified Mann Assessment of Swallowing Ability (MMASA), Munich Dysphagia Test-Parkinson’s Disease (MDT-PD), and the Mayo Dysphagia Questionnaire (MDQ) are tedious and time-consuming; and the Dysphagia in Multiple Sclerosis (DYMUS), M.D. Anderson Dysphagia Inventory (MDADI), and MetroHealth Dysphagia Screen (MDS) questionnaires are only applicable to specific patient groups [11, 32, 33].
The Eating Assessment Tool-10 (EAT-10) is a dysphagia screening tool developed in 2008 by Belafsky et al. [11] to identify people at high risk of swallowing disorders. EAT-10 is currently being used in clinical settings worldwide, has been translated into Chinese [34], Spanish [35], Swedish [36], Italian [37], Brazilian Portuguese [38], European Portuguese [39], Hebrew [40], Greek [41], French [42], and other languages. It is a 10-item self-assessment scale that patients can complete in a short period of time. Each item corresponds to 5 levels of difficulty from “no problem” to “serious problem,” with a total score of 0 to 40. EAT-10 has good internal consistency and intraclass correlation coefficients (ICCs) [11, 34, 36, 37, 39, 40, 42,43,44,45,46,47]. It has been proven to be useful in screening dysphagia in the oropharyngeal and esophageal phases [11, 44] and for swallowing disorders in a healthy population. There is a good correlation between the EAT-10 score and pharyngeal residual, penetration, and aspiration by FEES and VFSS [40, 48]. All of a patient’s symptoms can be assessed in 3 min [41, 44]. EAT-10 is low-risk for general practitioners, nurses, and other healthcare providers and requires no specialized training. However, there are differences in the reported sensitivity and specificity of EAT-10, and the best cutoff value remains controversial [44, 47, 49]. This study investigated the diagnostic accuracy of EAT-10 screening for dysphagia by meta-analysis, when FEES or VFSS were used as the gold standard and identified the optimal EAT-10 cutoff value to better guide clinical application.
Methods
The protocol was registered prospectively on PROSPERO (CRD42022300293).
Study Search
We searched PubMed, Web of Science, EMBASE, the Cochrane Library, CNKI, WANFANG, and VIP from May 2008 (when EAT-10 was developed) to March 2022. The search terms were as follows: (“dysphagia” OR “deglutition disorders” OR “swallowing”) AND (“EAT-10” OR “Eating Assessment Tool”). We also manually searched the references for the selected articles to obtain other eligible studies.
Study Selection
The inclusion criteria were as follows: age ≥ 18 years, clear consciousness, ability to understand and cooperate to complete the questionnaire, screened by EAT-10 with a cutoff value of 2 or 3, diagnostic gold standard of FEES or VFSS, dysphagia grade of Penetration-Aspiration Scale (PAS) ≥ 2, and the results of the study can be used to directly or indirectly obtain true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) rates.
The exclusion criteria were as follows: conference papers, case reports, letters, and reviews; significant missing data; data in the four-compartment table were not available; the authors did not reply to contact; the quality of the study was poor; the quality evaluation grade was C; or errors in statistical methods.
Data Extraction
Two trained researchers independently screened, evaluated, and extracted the literature and then cross-checked the literature. In cases of disagreement, the third researcher decided on whether to include the study. Researchers completed preliminary screening of the literature by reading the title, abstract, and keywords of the literature. After reading full texts and determining the literature to be included, the following data were extracted: first author, country, year of publication, study design, number of patients, study population, reference standard, cutoff value, and TP, FP, TN, and FN rates. For incomplete studies, the original author was contacted to the greatest extent possible.
Quality Assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to assess the quality of the included literature by two researchers and a third reassessed studies in cases of disagreement. There were 4 studies with 10 different evaluation results. The risk of bias was evaluated in four parts: patient selection, index test, reference standard, and flow and timing. The QUADAS-2 scale has a total of 14 items, each with three evaluation standards: “yes” indicates that the standard is met, “no” indicates that the study does not meet the standard or it is not mentioned, and “unclear” indicates that relevant information cannot be obtained from the literature. Finally, the literature was assessed as high, medium, or low quality.
Data Analysis
Meta-Disk 1.4 [50] was used to test heterogeneity. The diagnostic threshold effect test was performed using Spearman’s correlation coefficient; if P > 0.05, it was considered that there was no threshold effect, and each date could be combined. The Cochran–Q test for the diagnostic odds ratio (DOR) was used to detect heterogeneity caused by non-threshold effects. I2 > 50% suggested considerable heterogeneity. The area under the curve (AUC) and Q index were calculated to evaluate the diagnostic accuracy of EAT-10 at cutoff values of 2 and 3. RevMan 5.3 [51] was used to draw summary receiver operating characteristic (SROC) curves. TP, FP, TN, and FN values were input into STATA 16.0 (StataCorp, College Station, TX) to calculate the summary sensitivities, specificities, positive and negative likelihood ratios (PLRs and NLRs), and summary DORs with 95% confidence intervals (CIs).
Results
Study Selection
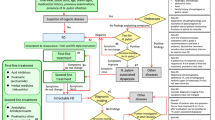
A total of 955 related studies were preliminarily identified and 734 duplicate studies were excluded. The researchers read the titles and abstracts for preliminary screening of the literature, carefully read the full text, and evaluated the literature quality. Finally, seven studies were included. A literature screening flowchart is shown in Fig. 1.
Study Characteristics
The meta-analysis included 1064 subjects from 7 countries, including healthy people and people at high risk for dysphagia, such as those with PD [52], stroke [34], amyotrophic lateral sclerosis (ALS) [53], aging [49], neurodegenerative diseases [36, 49], demyelinating disease [44], and gastroesophageal reflux disease [44]. Four studies [34, 44, 49, 53] used VFSS and three studies [36, 47, 52] used FEES as the gold standard for reporting the sensitivity and specificity of EAT-10. Cross-sectional studies [34, 36, 44, 52, 53] and case–control studies [47, 49] were included. The sensitivity and specificity with an EAT-10 cutoff of 2 were 81.48–93.58% and 36.36–82.35% and 48.65–98.25% and 52.73–98.25% with a cutoff of 3, respectively. The specific features of the literature are listed in Table 1.
Study Quality
Two researchers independently evaluated all included studies according to QUADAS-2 [54]. QUADAS-2 consists of 3 grades and 14 items (excluding the 3 non-essential items suggested by Cochrane). Among the seven studies, two were high quality and five were medium quality. A brief description of study quality is provided in Table 2. The results of the risk of bias assessment based on the Cochrane checklist are shown in Fig. 2. The study by Giraldo-Cadavid et al. [44] had an ambiguous risk with respect to patient selection and did not indicate whether patients were included consecutively. The studies by Schlickewei et al. [52] and Rofes et al. [49] were all blinded, but those by Järvenpää et al. [47], Rumi et al. [34], Plowman et al. [53], Möller et al. [36], and Giraldo-Cadavid et al. [44] did not explain whether blinding was used in the interpretation of the gold standard, and ambiguous attention was given to the gold standard. The reference standard for all studies correctly classified the target condition and used a PSA ≥ 2 to define dysphagia in advance. All studies avoided a case–control design.
Meta-Analysis
Heterogeneity Test
When the EAT-10 cutoff values were 2 and 3, the Spearman’s correlation coefficients were 0.500 (P = 0.667) and 0.500 (P = 0.253), respectively, and there was no heterogeneity caused by the threshold effect. The I2 statistic was 12.5% (P = 0.32) using a cutoff value of 2, suggesting that there was no heterogeneity caused by a non-threshold effect, and it was 92.8% (P < 0.05) when using a cutoff value of 3. The heterogeneity of included studies was large, and a random-effects model was adopted.
Diagnostic Accuracy of EAT-10
Using an EAT-10 cutoff value of 2, the pooled sensitivity, specificity, PLR, NLR, and DOR were 0.89 (95% CI 0.82–0.93), 0.59 (95% CI 0.39–0.77), 2.17 (95% CI 1.38–3.42), 0.19 (95% CI 0.13–0.29), and 11.49 (95% CI 5.86–22.53), respectively (Fig. 3). Using a cutoff value of 3, these values were 0.85 (95% CI 0.68–0.94), 0.82 (95% CI 0.65–0.92), 4.84 (95% CI 1.72–13.50), 0.18 (95% CI 0.07–0.46), and 26.24 (95% CI 5.06–135.95), respectively (Fig. 4). The diagnostic accuracy of EAT-10 at each cutoff value is described in Table 3.
The SROC curves with cutoff values of 2 and 3 are plotted in Fig. 5, showing that the curve with a cutoff value of 3 is better than the one with a cutoff value of 2. Using a cutoff value of 2, the Q statistic was 0.80 and the AUC was 0.87 (95% CI 0.82–0.93). Using a cutoff value of 3, the Q statistic was 0.84 and the AUC was 0.90 (95% CI 0.88–0.93).
Discussion
Dysphagia can cause serious complications, and early screening can help intervene in high-risk groups, effectively reducing dehydration, malnutrition, and aspiration pneumonia. However, even in hospitals, swallowing disorders are often ignored, dysphagia is often not reported by patients and ignored by clinicians, and the true prevalence of dysphagia may be higher than reported [55].
EAT-10 has been translated into various languages. The internal consistency between different versions (Cronbach’s α) ranges from 0.84 to 0.96 and ICCs range from 0.70 to 1.00. The internal consistency and retest reliability of the different EAT-10 versions are shown in Table 4.
EAT-10 has good predictive value for swallowing disorders caused by various diseases. EAT-10 scores in patients with head and neck cancer with dysphagia were significantly higher than those in patients without dysphagia (P < 0.05) [41]. When the EAT-10 cutoff value was 15, the sensitivity and specificity in predicting aspiration for nervous system diseases [56] was 81.0% and 58.0%, respectively. Using a cutoff value of 9, unilateral vocal fold paralysis aspiration [57] in patients was predicted with a sensitivity of 77.8% and specificity of 73.1%. EAT-10 scores can also predict the presence of aspiration in patients with ALS and chronic obstructive pulmonary disease (COPD). ALS [53] had a sensitivity of 86%, specificity of 76%, and a likelihood ratio of 3.1. Stable COPD [58] was observed in 91.67% and 77.78% of patients, and the DOR was 38.50. However, the ability of EAT-10 to predict penetration and aspiration in patients with PD was disappointing, with a sensitivity of only 58% at a cutoff of 6 [52]. This is because patients with PD usually have decreased laryngopharyngeal sensitivity, are unable to notice whether the residue after swallowing is removed, and the threshold of the cough reflex increases [59, 60], thus affecting the answers to questions 3–9. The pediatric version of EAT‐10 has a cutoff value of 4, with a sensitivity and specificity of 91.3% and 98.8%, respectively, which could effectively distinguish children with dysphagia from healthy children. EAT-10 has been found to correlate strongly (r = 0.41, P < 0.001) with the FEES PAS score [46].
Although many studies have reported the diagnostic accuracy of EAT-10, there is still no consensus on the best cutoff value for the diagnosis of dysphagia. Giraldo-Cadavid et al. [44] and Rofes et al. [49] believed that using 2 as the EAT-10 cutoff value had higher sensitivity and could avoid missing true diagnoses of any patient with dysphagia, but it could also increase the rate of misdiagnosis. Belafsky et al. [11], the authors of the original EAT-10, and many other researchers [36, 37, 39, 47, 53] suggested using 3 as the cutoff value because it has a better balance between sensitivity and specificity and a higher diagnostic accuracy. Giraldo-Cadavid et al. [44] also reported 94.3% sensitivity and 49.5% specificity when using 4 as an EAT-10 cutoff value. In this study, a meta-analysis of the most controversial values – 2 and 3 – was conducted to calculate their diagnostic performance and to determine the best cutoff value for EAT-10 in predicting patients with swallowing disorders.
There were 7 studies included, involving 1064 subjects from 7 different countries. Two studies were high quality and five studies were medium quality, with no significant risk deviation. Sensitivity and specificity are important indexes to evaluate screening tools. Sensitivity refers to the ability to screen out patients with illness or related symptoms and specificity is the ability to exclude patients without disease or related symptoms. The summary sensitivity of a cutoff of 2 was 4% higher than that of 3, but the specificity of a cutoff of 2 was 23% lower than that of 3 at the cost of greatly increasing the misdiagnosis rate. In this study, the DOR of the cutoff value of 3 is significantly higher than that of 2, indicating that using 3 as the EAT-10 cutoff value has a better discrimination effect for dysphagia. This screening tool should have high recognition ability for swallowing disorders. Although sensitivity is the primary indicator to consider for the screening tool, the diagnostic value of 3 is higher than that of 2 when specificity is taken into account. The AUC of the SROC curve reflects the accuracy of diagnostic tests. Our results show that EAT-10 has good accuracy for a cutoff value of 2 or 3, which can be applied in clinical screening of people with dysphagia. SROC curves consider both specificity and sensitivity. This study shows that an EAT-10 cutoff value of 3 has a diagnostic accuracy better than a cutoff of 2.
In this study, some factors affected the results of the sensitivity and specificity, which may arise from several factors. First, cultural differences can affect the clinical application of EAT-10. Shapira-Galitz et al. [27] found that the Hebrew version of EAT-10 was different in patients’ use of their mother tongue. The mean EAT-10 score of the dysphagia group was higher than that of English speakers, but lower than that of Italian, Spanish, or Swedish speakers and especially Japanese speakers. Even between the two Hebrew versions, there was a difference in the mean scores of the dysphagia group [43]. Languages that tend to score higher will show higher sensitivity and lower specificity in the EAT-10 diagnostic accuracy validation. Second, patients with obvious major psychiatric or cognitive disorders were excluded, as they would be unable to utilize the questionnaire. Mölle et al. [36] did not consider the cognitive status of their patients. As such, their study design does not accurately reflect the individual items in the EAT-10. Thus, when compared to the gold standard, the EAT-10 score results differ significantly from the gold standard, and the diagnostic accuracy of the resulting questionnaire is lower than the true values. Third, as penetration and aspiration are usually sporadic, VFSS and FEES may produce FN results and fail to identify the risk of penetration and aspiration in some high-risk groups [48, 61, 62], affecting EAT-10 sensitivity and specificity; in these studies, the sensitivity, specificity, and positive prediction values obtained were lower than true values. Fourth, the type of disease and the choice of the gold standard also affect the sensitivity and specificity of EAT-10. EAT-10 cannot accurately indicate patients with dysphagia caused by decreased sensitivity, such as those with PD [52]. The EAT-10 questionnaire completed by these patients may yield FN results, and so the sensitivity, positive predictive value, and negative predictive value obtained would be reduced compared to the true values. When patients with COPD are evaluated for swallowing disorders, FEES has better reliability than VFSS because it can better evaluate the pharyngeal mucosa, laryngopharyngeal sensitivity, and vocal cords. PAS scores in FEES are often higher than those with VFSS [62, 63]. Using VFSS as the gold standard, the sensitivity, specificity, and positive predictive value obtained would be somewhat lower compared to FEES.
EAT-10 also has good psychometric properties and reliability [11, 43,44,45, 64]; however, Cordier et al. [64] conducted Rasch analysis on EAT-10 and found that it has significant weaknesses in construction validity, including item redundancy, the lack of easy and difficult items, and different thresholds of rating scale categories. The second question (“My swallowing problem interferes with my ability to go out for meals”) is difficult to answer for some patients who have been hospitalized for a long time due to diseases and only present or are diagnosed with dysphagia during hospitalization. Item 2 may not be suitable for screening patients with dysphagia [34]. Therefore, EAT-10 needs to be improved for better clinical applications and research. (1) When designing the questionnaire, the questions should be designed from easy to difficult and from concrete to abstract. The EAT-10 can adjust the order of the items and consider putting items 2, 7, and 10 at the end of the questionnaire. (2) A Rasch analysis of the EAT-10 reveals that items 2, 7, and 10 do not contribute to the overall construct [64]. We should consider removing these three items from the EAT-10. (3) The second question (“My swallowing problem interferes with my ability to go out for meals”) is difficult to answer for some patients who have been hospitalized for a long time due to diseases and only present or are diagnosed with dysphagia during hospitalization. Item 2 may be unsuitable for screening patients with dysphagia [34]. “Go out’ focuses more on a patient’s mobility than on the impact of swallowing difficulties on life interactions. We can consider replacing item 2 with ‟My swallowing problem has affected my ability to eat with others.” (4) The Rasch analysis [64] also revealed that the EAT-10 was unable to form a second dimension that would allow further differentiation according to patient ability. Oral phase, esophageal phase, airway protection, and other related items can be considered in the EAT-10. Moreover, it is not suitable for swallowing disorders caused by decreased sensitivity [52].
Conclusion
In summary, EAT-10 has been translated into many languages and is widely used in clinical practice. Although EAT-10 has some structural validity defects, it has good internal consistency, ICCs, and good psychometric properties. There was a linear correlation between EAT-10 and PAS scores, which could also reflect the severity of dysphagia to a certain extent. Using 2 and 3 as cutoff values showed good diagnostic performance. EAT-10 can be used as a preliminary screening tool for dysphagia. However, the diagnostic accuracy with a cutoff of 3 is higher, which can not only screen most high-risk groups of swallowing disorders but also avoid a high misdiagnosis rate. Therefore, a cutoff of 3 is recommended as the best cutoff value for EAT-10.
References
Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116(2):455–78. https://doi.org/10.1016/s0016-5085(99)70144-7.
Triggs J, Pandolfino J. Recent advances in dysphagia management. F1000Research. 2019. https://doi.org/10.12688/f1000research.18900.1.
Estupiñán Artiles C, Regan J, Donnellan C. Dysphagia screening in residential care settings: a scoping review. Int J Nurs Stud. 2021;114:103813. https://doi.org/10.1016/j.ijnurstu.2020.103813.
Clavé P, Rofes L, Carrión S, Ortega O, Cabré M, Serra-Prat M, Arreola V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66. https://doi.org/10.1159/000339986.
Clavé P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol. 2015;12(5):259–70. https://doi.org/10.1038/nrgastro.2015.49.
Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287–98. https://doi.org/10.2147/cia.S23404.
Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000;10(5):380–6. https://doi.org/10.1159/000016094.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–63. https://doi.org/10.1161/01.STR.0000190056.76543.eb.
Ebihara S, Sekiya H, Miyagi M, Ebihara T, Okazaki T. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J Thorac Dis. 2016;8(3):632–9. https://doi.org/10.21037/jtd.2016.02.60.
Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311–5. https://doi.org/10.1016/j.parkreldis.2011.11.006.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. https://doi.org/10.1177/000348940811701210.
Chu LW, Pei CK. Risk factors for early emergency hospital readmission in elderly medical patients. Gerontology. 1999;45(4):220–6. https://doi.org/10.1159/000022091.
Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–9. https://doi.org/10.1001/archoto.2010.129.
Bray BD, Smith CJ, Cloud GC, Enderby P, James M, Paley L, Tyrrell PJ, Wolfe CD, Rudd AG. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J Neurol Neurosurg Psychiatry. 2017;88(1):25–30. https://doi.org/10.1136/jnnp-2016-313356.
Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin-Harris B, Simpson K. The one-year attributable cost of post-stroke dysphagia. Dysphagia. 2014;29(5):545–52. https://doi.org/10.1007/s00455-014-9543-8.
Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, Leners JC, Masiero S, Mateos-Nozal J, Ortega O, Smithard DG, Speyer R, Walshe M. European Society for Swallowing Disorders—European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. 2016;11:1403–28. https://doi.org/10.2147/cia.S107750.
Fattori B, Giusti P, Mancini V, Grosso M, Barillari MR, Bastiani L, Molinaro S, Nacci A. Comparison between videofluoroscopy, fiberoptic endoscopy and scintigraphy for diagnosis of oro-pharyngeal dysphagia. Acta Otorhinolaryngol Ital. 2016;36(5):395–402. https://doi.org/10.14639/0392-100x-829.
Moro L, Cazzani C. Dynamic swallowing study and radiation dose to patients. Radiol Med. 2006;111(1):123–9. https://doi.org/10.1007/s11547-006-0013-8.
McLean D, Smart R, Collins L, Varas J. Thyroid dose measurements for staff involved in modified barium swallow exams. Health Phys. 2006;90(1):38–41. https://doi.org/10.1097/01.hp.0000174812.97049.0e.
Hayes A, Alspaugh JM, Bartelt D, Campion MB, Eng J, Gayler BW, Henkel SE, Jones B, Lingaraj A, Mahesh M, Rostkowski M, Smith CP, Haynos J. Radiation safety for the speech-language pathologist. Dysphagia. 2009;24(3):274–9. https://doi.org/10.1007/s00455-008-9201-0.
Wright RE, Boyd CS, Workman A. Radiation doses to patients during pharyngeal videofluoroscopy. Dysphagia. 1998;13(2):113–5. https://doi.org/10.1007/pl00009554.
Chan CB, Chan LK, Lam HS. Scattered radiation level during videofluoroscopy for swallowing study. Clin Radiol. 2002;57(7):614–6. https://doi.org/10.1053/crad.2001.0904.
Weir KA, McMahon SM, Long G, Bunch JA, Pandeya N, Coakley KS, Chang AB. Radiation doses to children during modified barium swallow studies. Pediatr Radiol. 2007;37(3):283–90. https://doi.org/10.1007/s00247-006-0397-6.
Zammit-Maempel I, Chapple CL, Leslie P. Radiation dose in videofluoroscopic swallow studies. Dysphagia. 2007;22(1):13–5. https://doi.org/10.1007/s00455-006-9031-x.
Chau KH, Kung CM. Patient dose during videofluoroscopy swallowing studies in a Hong Kong public hospital. Dysphagia. 2009;24(4):387–90. https://doi.org/10.1007/s00455-009-9214-3.
Crawley MT, Savage P, Oakley F. Patient and operator dose during fluoroscopic examination of swallow mechanism. Br J Radiol. 2004;77(920):654–6. https://doi.org/10.1259/bjr/22832251.
Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19(4):769–85. https://doi.org/10.1016/j.pmr.2008.06.004.
Bonilha HS, Humphries K, Blair J, Hill EG, McGrattan K, Carnes B, Huda W, Martin-Harris B. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85. https://doi.org/10.1007/s00455-012-9415-z.
Madden C, Fenton J, Hughes J, Timon C. Comparison between videofluoroscopy and milk-swallow endoscopy in the assessment of swallowing function. Clin Otolaryngol Allied Sci. 2000;25(6):504–6. https://doi.org/10.1046/j.1365-2273.2000.00385.x.
Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–6. https://doi.org/10.1161/01.STR.0000177529.86868.8d.
Magalhães Junior HV, Pernambuco LA, Lima KC, Ferreira MAF. Screening for oropharyngeal dysphagia in older adults: a systematic review of self-reported questionnaires. Gerodontology. 2018. https://doi.org/10.1111/ger.12333.
Yu L, Wang J, Wang MH, Zhang DY, Yang W, Lei S. Research progress on dysphagia screening scale for the aged. Chin J Mod Nurs. 2020;26(6):711–6. https://doi.org/10.3760/cma.j.issn.1674-2907.2020.06.003.
Li H, Feng H, Chen HJ, Wang RM. Application progress of dysphagia screening tool in elderly care services. Chin J Rehabil Med. 2020;35(3):356–60. https://doi.org/10.3969/j.issn.1001-1242.2020.03.022.
Rumi W, Xuehong X, Changjie Z, Yongmei F. Reliability and validity of the Chinese Eating Assessment Tool (EAT-10) in evaluation of acute stroke patients with dysphagia. J Cent South Univ. 2015;40(12):1391–9. https://doi.org/10.11817/j.issn.1672-7347.2015.12.017.
Burgos R, Sarto B, Segurola H, Romagosa A, Puiggrós C, Vázquez C, Cárdenas G, Barcons N, Araujo K, Pérez-Portabella C. Translation and validation of the Spanish version of the EAT-10 (Eating Assessment Tool-10) for the screening of dysphagia. Nutr Hosp. 2012;27(6):2048–54. https://doi.org/10.3305/nh.2012.27.6.6100.
Möller R, Safa S, Östberg P. Validation of the Swedish translation of eating assessment tool (S-EAT-10). Acta Otolaryngol. 2016;136(7):749–53. https://doi.org/10.3109/00016489.2016.1146411.
Schindler A, Mozzanica F, Monzani A, Ceriani E, Atac M, Jukic-Peladic N, Venturini C, Orlandoni P. Reliability and validity of the Italian Eating Assessment Tool. Ann Otol Rhinol Laryngol. 2013;122(11):717–24. https://doi.org/10.1177/000348941312201109.
Gonçalves MI, Remaili CB, Behlau M. Cross-cultural adaptation of the Brazilian version of the Eating Assessment Tool—EAT-10. Codas. 2013;25(6):601–4. https://doi.org/10.1590/s2317-17822013.05000012.
Nogueira DS, Ferreira PL, Reis EA, Lopes IS. Measuring outcomes for dysphagia: validity and reliability of the European Portuguese eating Assessment Tool (P-EAT-10). Dysphagia. 2015;30(5):511–20. https://doi.org/10.1007/s00455-015-9630-5.
Shapira-Galitz Y, Yousovich R, Halperin D, Wolf M, Lahav Y, Drendel M. Does the Hebrew Eating Assessment Tool-10 correlate with pharyngeal residue, penetration and aspiration on fiberoptic endoscopic examination of swallowing? Dysphagia. 2019;34(3):372–81. https://doi.org/10.1007/s00455-018-9964-x.
Printza A, Kyrgidis A, Pavlidou E, Triaridis S, Constantinidis J. Reliability and validity of the Eating Assessment Tool-10 (Greek adaptation) in neurogenic and head and neck cancer-related oropharyngeal dysphagia. Eur Arch Otorhinolaryngol. 2018;275(7):1861–8. https://doi.org/10.1007/s00405-018-5001-9.
Lechien JR, Cavelier G, Thill MP, Huet K, Harmegnies B, Bousard L, Blecic S, Vanderwegen J, Rodriguez A, Dequanter D. Validity and reliability of the French version of Eating Assessment Tool (EAT-10). Eur Arch Otorhinolaryngol. 2019;276(6):1727–36. https://doi.org/10.1007/s00405-019-05429-1.
Abu-Ghanem S, Schechter M, Flesh-Eyni H, Litwin L, Makai E, Oestreicher-Kedem Y, Yehuda M. Validation of the Hebrew Version of the Eating Assessment Tool-10 (H-EAT-10). Folia Phoniatr Logop. 2016;68(6):261–7. https://doi.org/10.1159/000484557.
Giraldo-Cadavid LF, Gutiérrez-Achury AM, Ruales-Suárez K, Rengifo-Varona ML, Barros C, Posada A, Romero C, Galvis AM. Validation of the Spanish Version of the Eating Assessment Tool-10 (EAT-10spa) in Colombia. A blinded prospective cohort study. Dysphagia. 2016;31(3):398–406. https://doi.org/10.1007/s00455-016-9690-1.
Demir N, Serel Arslan S, İnal Ö, Karaduman AA. Reliability and validity of the Turkish Eating Assessment Tool (T-EAT-10). Dysphagia. 2016;31(5):644–9. https://doi.org/10.1007/s00455-016-9723-9.
Serel Arslan S, Demir N, Karaduman AA, Belafsky PC. The Pediatric Version of the Eating Assessment Tool: a caregiver administered dyphagia-specific outcome instrument for children. Disabil Rehabil. 2018;40(17):2088–92. https://doi.org/10.1080/09638288.2017.1323235.
Järvenpää P, Kuuskoski J, Pietarinen P, Markkanen-Leppänen M, Freiberg H, Ruuskanen M, Rekola J, Ilmarinen T, Kinnari TJ, Autio TJ, Penttilä E, Muttilainen MS, Laaksonen A, Oksanen L, Geneid A, Aaltonen LM. Finnish version of the Eating Assessment Tool (F-EAT-10): a valid and reliable patient-reported outcome measure for dysphagia evaluation. Dysphagia. 2021. https://doi.org/10.1007/s00455-021-10362-9.
Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The ability of the 10-Item Eating Assessment Tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(5):351–4. https://doi.org/10.1177/0003489414558107.
Rofes L, Arreola V, Mukherjee R, Clavé P. Sensitivity and specificity of the Eating Assessment Tool and the Volume-Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–65. https://doi.org/10.1111/nmo.12382.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. https://doi.org/10.1186/1471-2288-6-31.
Schmidt L, Shokraneh F, Steinhausen K, Adams CE. Introducing RAPTOR: RevMan parsing tool for reviewers. Syst Rev. 2019;8(1):151. https://doi.org/10.1186/s13643-019-1070-0.
Schlickewei O, Nienstedt JC, Frank U, Fründt O, Pötter-Nerger M, Gerloff C, Buhmann C, Müller F, Lezius S, Koseki JC, Pflug C. The ability of the eating assessment tool-10 to detect penetration and aspiration in Parkinson’s disease. Eur Arch Otorhinolaryngol. 2021;278(5):1661–8. https://doi.org/10.1007/s00405-020-06377-x.
Plowman EK, Tabor LC, Robison R, Gaziano J, Dion C, Watts SA, Vu T, Gooch C. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2016;28(1):85–90. https://doi.org/10.1111/nmo.12700.
Reitsma JB, Moons KG, Bossuyt PM, Linnet K. Systematic reviews of studies quantifying the accuracy of diagnostic tests and markers. Clin Chem. 2012;58(11):1534–45. https://doi.org/10.1373/clinchem.2012.182568.
Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–46. https://doi.org/10.1007/s00455-001-0113-5.
Arslan SS, Demir N, Kılınç HE, Karaduman AA. The Ability of the Eating Assessment Tool-10 to detect aspiration in patients with neurological disorders. J Neurogastroenterol Motil. 2017;23(4):550–4. https://doi.org/10.5056/jnm16165.
Zuniga SA, Ebersole B, Jamal N. Utility of Eating Assessment Tool-10 in predicting aspiration in patients with unilateral vocal fold paralysis. Otolaryngol Head Neck Surg. 2018;159(1):92–6. https://doi.org/10.1177/0194599818762328.
Regan J, Lawson S, De Aguiar V. The Eating Assessment Tool-10 predicts aspiration in adults with stable chronic obstructive pulmonary disease. Dysphagia. 2017;32(5):714–20. https://doi.org/10.1007/s00455-017-9822-2.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, Adler CH, Shill HA, Caviness JN, Samanta JE, Sue LI, Beach TG. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72(7):614–23. https://doi.org/10.1097/NEN.0b013e3182965886.
Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson disease. Chest. 2014;146(5):1294–9. https://doi.org/10.1378/chest.14-0066.
Jang DH, Choi KH, Kim DH, Lim CM, Kim JS. Comparison between the radionuclide salivagram and videofluoroscopic swallowing study methods for evaluating patients with aspiration pneumonia. Ann Nucl Med. 2013;27(3):247–52. https://doi.org/10.1007/s12149-012-0680-6.
Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117(10):1723–7. https://doi.org/10.1097/MLG.0b013e318123ee6a.
Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17(4):308–15. https://doi.org/10.1007/s00455-002-0073-4.
Cordier R, Joosten A, Clavé P, Schindler A, Bülow M, Demir N, Arslan SS, Speyer R. Evaluating the Psychometric Properties of the Eating Assessment Tool (EAT-10) Using Rasch Analysis. Dysphagia. 2017;32(2):250–60. https://doi.org/10.1007/s00455-016-9754-2.
Acknowledgements
Diagnostic accuracy of the Eating Assessment Tool-10 (EAT-10) in screening dysphagia: a meta-analysis registered in the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42020164655.
Funding
This work was supported by [Shandong Traditional Chinese Medicine Science and Technology Project in 2021] Grant Number [2021M155], [Weifang soft science research project in 2021] Grant Number [2021RKX071], and [The School Innovation and Entrepreneurship Training Program of Weifang Medical University in 2021] Grant Number [x2021137].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Pp., Yuan, Y., Lu, Dz. et al. Diagnostic Accuracy of the Eating Assessment Tool-10 (EAT-10) in Screening Dysphagia: A Systematic Review and Meta-Analysis. Dysphagia 38, 145–158 (2023). https://doi.org/10.1007/s00455-022-10486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-022-10486-6