Abstract
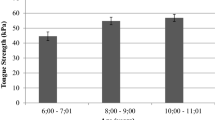
Age-related changes in muscle composition and function are often treated using exercise, including muscles of the tongue to treat swallowing impairments (dysphagia). Although tongue exercise is commonly prescribed, optimal tongue exercise doses have not been determined. The purpose of this study was to evaluate effects of varying tongue exercise frequency on tongue force, genioglossus muscle fiber size, composition and metabolism, and swallowing in a rat model. We randomized 41 old and 40 young adult Fischer 344/Brown Norway rats into one of four tongue exercise groups: 5 days/week; 3 days/week; 1 day/week; or sham. Tongue force was higher following all exercise conditions (vs sham); the 5 day/week group had the greatest change in tongue force (p < 0.001). There were no exercise effects on genioglossus (GG) fiber size or MyHC composition (p > 0.05). Significant main effects for age showed a greater proportion of Type I fibers in (p < 0.0001) and increased fiber size of IIa fibers (p = 0.026) in old. There were no significant effects of citrate synthase activity or PGC-1α expression. Significant differences were found in bolus speed and area (size), but findings were potentially influenced by variability. Our findings suggest that tongue force is influenced by exercise frequency; however, these changes were not reflected in characteristics of the GG muscle assayed in this study. Informed by findings of this study, future work in tongue dose optimization will be required to provide better scientific premise for clinical treatments in humans.
Similar content being viewed by others
References
Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112(4):589–602.
Roy N, et al. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–65.
Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. Washington, DC: US Census Bureau; 2014. p. 25–1140.
Robbins J, et al. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–9.
Cook I, et al. (1994) Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol Gastrointest Liver Physiol. 1994;266(6):972–7.
Martin BJ, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9(1):1–6.
Mcconnel F. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98(1):71–8.
Tamine K, et al. Age-related changes in tongue pressure during swallowing. J Dent Res. 2010;89(10):1097–101.
Nicosia MA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A. 2000;55(11):M634–M640640.
Maeda K, Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30(1):80–7.
Yoshida M, et al. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21(1):61–5.
Namasivayam AM, Steele CM, Keller H. The effect of tongue strength on meal consumption in long term care. Clin Nutr. 2016;35(5):1078–83.
Altman KW, Yu G-P, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–9.
Cabre M, et al. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
Cichero JA, Altman KW. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources, in Stepping stones to living well with dysphagia. Basel: Karger Publishers; 2012. p. 1–11.
Eslick GD, Talley N. Dysphagia: epidemiology, risk factors and impact on quality of life–a population-based study. Aliment Pharmacol Ther. 2008;27(10):971–9.
Humbert IA, Robbins J. Dysphagia in the elderly. Phys Med Rehabil Clin N Am. 2008;19(4):853–66.
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s.
Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci. 1988;84(2):275–94.
Evans WJ, Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A. 1995;50(Special Issue):11–6.
Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81(11):1810–6.
Alnaqeeb M, Goldspink G. Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat. 1987;153:31.
Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–42.
Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol. 2002;27(1):19–411.
Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854(1):92–101.
Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128(2):351S–5S.
Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–27.
Frontera WR, Zayas AR, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Phys Med Rehabil Clin. 2012;23(1):201–7.
Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45(5):397–458.
Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol. 1997;22(4):307–27.
Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Technol. 2000;50(6):500–9.
Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223.
Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012. https://doi.org/10.1155/2012/194821.
Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci. 1996;93(26):15364–9.
Miquel J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch Gerontol Geriatr. 1991;12(2–3):99–117.
Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–8.
Boffoli D, et al. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226(1):73–82.
Carter HN, Chen CC, Hood DA. Mitochondria, muscle health, and exercise with advancing age. Physiology. 2015;30(3):208–23.
Adhihetty PJ, et al. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88(1):99–107.
Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–51.
Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24.
Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90.
Tipton CM, Medicine ACOS. ACSM's advanced exercise physiology. Philadelphia: Lippincott Williams & Wilkins; 2006.
Derbré F, et al. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age. 2012;34(3):669–79.
Gibala MJ, et al. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106(3):929–34.
Tanaka K, et al. Preventive effects of electrical stimulation on inflammation-induced muscle mitochondrial dysfunction. Acta Histochem. 2016;118(5):464–70.
Kawai N, et al. Adaptation of rat jaw muscle fibers in postnatal development with a different food consistency: an immunohistochemical and electromyographic study. J Anat. 2010;216(6):717–23.
Wright DC, et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282(1):194–9.
Brealey D, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. The Lancet. 2002;360(9328):219–23.
Shepherd D, Garland P. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969;114(3):597–610.
Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90(3):1137–57.
Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15(1):97–117.
Srere P. [1] Citrate synthase: [EC 4.1. 3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 1969;13:3–11.
Robbins J, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–8.
Lazarus C. Tongue strength and exercise in healthy individuals and in head and neck cancer patients. Seminars in speech and language. New York: Thieme Medical Publishers; 2006.
Oh JC. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia. 2015;30(3):315–20.
Park J-S, Kim H-J, Oh D-H. Effect of tongue strength training using the Iowa oral performance instrument in stroke patients with dysphagia. J Phys Ther Sci. 2015;27(12):3631–4.
Robbins J, et al. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53(9):1483–9.
Rogus-Pulia N, et al. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J Am Geriatr Soc. 2016;64(2):417–24.
Robbins J, et al. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A. 1995;50(5):M257–M262262.
Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–65.
Langmore SE, Pisegna JM. Efficacy of exercises to rehabilitate dysphagia: a critique of the literature. Int J Speech Lang Pathol. 2015;17(3):222–9.
Ferguson B. ACSM’s guidelines for exercise testing and prescription 9th Ed. J Can Chiropractic Assoc. 2014;58(3):328.
Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3:735.
Krisciunas GP, et al. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia. 2012;27(4):538–49.
Virani A, et al. Effects of 2 different swallowing exercise regimens during organ-preservation therapies for head and neck cancers on swallowing function. Head Neck. 2015;37(2):162–70.
McKenna VS, et al. A systematic review of isometric lingual strength-training programs in adults with and without dysphagia. Am J Speech Lang Pathol. 2017;26(2):524–39.
Peterson MD, Rhea MR, Alvar BA. Applications of the dose-response for muscular strength development: areview of meta-analytic efficacy and reliability for designing training prescription. J Strength Cond Res. 2005;19(4):950–8.
Connor NP, et al. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–44.
Connor NP, et al. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51(4):818–27.
Kletzien H, et al. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol. 2013;114(4):472–81.
Schaser AJ, Ciucci MR, Connor NP. Cross-activation and detraining effects of tongue exercise in aged rats. Behav Brain Res. 2016;297:285–96.
Krekeler BN, Connor NP. Age-related changes in mastication are not improved by tongue exercise in a rat model. Laryngoscope. 2017;127(1):E29–e34.
Schaser AJ, et al. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2011;26(3):256–63.
Russell JA, et al. Videofluorographic assessment of deglutitive behaviors in a rat model of aging and Parkinson disease. Dysphagia. 2013;28(1):95–104.
Lenth RV (2006) Java applets for power and sample size [computer software]. 9.
Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. J Am Assoc Lab Anim Sci. 2000;39(6):9–17.
Cullins MJ, Connor NP. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve. 2017;56:E119–E12525.
Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE. 2012;7(4):e35273.
Schiaffino S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10(3):197–205.
Lucas CA, Kang LH, Hoh JF. Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun. 2000;272(1):303–8.
Eddinger TJ, Moss RL, Cassens RG. Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J Histochem Cytochem. 1985;33(10):1033–41.
Smith LR, Barton ER. SMASH-semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skeletal muscle. 2014;4(1):1.
Li R, Shen Y. An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013;92(13):747–51.
Eaton SL, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE. 2013;8(8):e72457.
Antharavally BS, et al. A high-affinity reversible protein stain for Western blots. Anal Biochem. 2004;329(2):276–80.
McLester JR, Bishop E, Guilliams M. Comparison of 1 day and 3 days per week of equal-volume resistance training in experienced subjects. J Strength Cond Res. 2000;14(3):273–81.
Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2010;42(5):902–14.
Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97(1):933–6.
Palmer PM, et al. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51(4):828–35.
Ota F, Connor NP, Konopacki R. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol. 2005;114(10):799–803.
Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52(2):438–44.
Bole C 2nd, Lessler MA. Electromyography of the genioglossus muscles in man. J Appl Physiol. 1966;21(6):1695–8.
Cunningham DP, Basmajian JV. Electromyography of genioglossus and geniohyoid muscles during deglutition. Anat Rec. 1969;165(3):401–9.
Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–88.
Spiering BA, et al. Resistance exercise biology. Sports Med. 2008;38(7):527–40.
Häkkinen K, Alen M, Komi P. Changes in isometric force-and relaxation-time, electromyographic and muscle fibre characteristics of human skeletal muscle during strength training and detraining. Acta Physiol Scand. 1985;125(4):573–85.
Cartee GD, et al. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23(6):1034–47.
Wang L, et al. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J Appl Physiol. 2011;111(5):1335–444.
Lysenko E, et al. Effect of combined aerobic and strength exercises on the regulation of mitochondrial biogenesis and protein synthesis and degradation in human skeletal muscle. Hum Physiol. 2016;42(6):634–44.
Granata C, et al. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. FASEB J. 2016;30(10):3413–23.
Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. 2016;101(1):17–22.
Schwarz NA, et al. Effect of resistance exercise intensity on the expression of PGC-1α isoforms and the anabolic and catabolic signaling mediators, IGF-1 and myostatin, in human skeletal muscle. Appl Physiol Nutr Metab. 2016;41(8):856–63.
Coffey VG, et al. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab. 2006;290(5):E849–E855855.
Alvehus M, et al. Metabolic adaptations in skeletal muscle, adipose tissue, and whole-body oxidative capacity in response to resistance training. Eur J Appl Physiol. 2014;114(7):1463–71.
Salvadego D, et al. Skeletal muscle oxidative function in vivo and ex vivo in athletes with marked hypertrophy from resistance training. J Appl Physiol. 2013;114(11):1527–35.
Tesch P, Komi P, Häkkinen K. Enzymatic adaptations consequent to long-term strength training. Int J Sports Med. 1987;8(S1):S66–S69.
Wang N, et al. Muscle fiber types of women after resistance training—quantitative ultrastructure and enzyme activity. Pflügers Arch. 1993;424(5–6):494–502.
Porter C, et al. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc. 2015;47(9):1922.
Horan M, Pichaud N, Ballard JW. Quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A. 2012;67(10):1022–35.
Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–5.
Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15(2):161–7.
Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci. 1998;95(3):861–8.
Kletzien H, Cullins MJ, Connor NP. Age-related alterations in swallowing biomechanics. Exp Gerontol. 2019;118:45–50.
Amri M, Lamkaden M, Car A. Activity of extrinsic tongue muscle during swallowing in sheep. Brain Res. 1989;503(1):141–3.
Napadow VJ, et al. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol Gastroint Liver Physiol. 1999;277(3):G695–G701.
Robbins J, et al. Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia. 2016;31(1):90–6.
Krekeler BN, et al. Patient adherence to dysphagia recommendations: a systematic review. Dysphagia. 2018;33(2):173–84.
Acknowledgements
This work was supported by NIH Grants 1F31AG059351-01, R01DC018071, R01DC008149, R01DC014358, R37CA225608. This manuscript was submitted in partial fulfillment of the requirements for the doctoral dissertation of the first author, BNK. The authors would like to acknowledge the help of Dr. John Russell during animal tissue harvest, Jared Cullen for his help in coordination of animal training, Drs. Heidi Kletzien. Tiffany Glass, and Miranda Cullins for their encouragement and support in the learning of these assays. The first author would like to thank and recognize her dissertation committee members for their valuable contributions to her training and feedback on this manuscript: Drs. Nicole Rogus-Pulia, Michelle R Ciucci, Gary Diffee, Catriona Steele, and Timothy McCulloch; and Dr. Glen Leverson for his statistical mentorship throughout her dissertation work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krekeler, B.N., Weycker, J.M. & Connor, N.P. Effects of Tongue Exercise Frequency on Tongue Muscle Biology and Swallowing Physiology in a Rat Model. Dysphagia 35, 918–934 (2020). https://doi.org/10.1007/s00455-020-10105-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10105-2