Abstract
Background
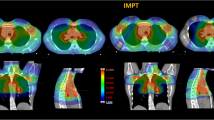
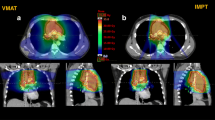
To investigate the role of intensity-modulated proton therapy (IMPT) compared to volumetric modulated arc therapy (VMAT) for the radiation treatment of thymoma cancer.
Methods
Twenty patients were retrospectively planned for IMPT [with (IMPT_R1 or IMPT_R2 according to the approach adopted) and without robust optimization] and VMAT. The results were compared according to dose-volume metrics on the clinical and planning target volumes (CTV and PTV) and the main organs at risk (heart, breasts, lungs, spinal cord and oesophagus). Estimates of the excess absolute risk (EAR) of secondary cancer induction were determined for the oesophagus, the breasts and the composite lungs. For the heart, the relative risk (RR) of chronic heart failure (CHF) was assessed.
Results
IMPT and VMAT plans resulted equivalent in terms of target coverage for both the CTV and the PTV. The CTV homogeneity index resulted in 0.03 ± 0.01 and 0.04 ± 0.01 for VMAT and all IMPT plans, respectively. The conformality index resulted in 1.1 ± 0.1 and 1.2 ± 0.1 for VMAT and all IMPT plans. The mean dose to the breasts resulted in 10.5 ± 5.0, 4.5 ± 3.4, 4.7 ± 3.5 and 4.6 ± 3.4 Gy for VMAT, IMPT, IMPT_R1 and IMPT_R2. For the lungs, the mean dose was 9.6 ± 2.3, 3.5 ± 1.5, 3.6 ± 1.6 and 3.8 ± 1.4 Gy; for the heart: 8.7 ± 4.4, 4.3 ± 1.9, 4.5 ± 2.0 and 4.4 ± 2.4 Gy and for the oesophagus 8.2 ± 3.5, 2.2 ± 3.4, 2.4 ± 3.6 and 2.5 ± 3.5 Gy. The RR for CHF was 1.6 ± 0.3 for VMAT and 1.3 ± 0.2 for IMPT (R1 or R2). The EAR was 3.6 ± 0.v vs 1.0 ± 0.6 or 1.2 ± 0.6 (excess cases/10,000 patients year) for the oesophagus; 17.4 ± 6.5 vs 5.7 ± 3.2 or 6.1 ± 3.8 for the breasts and 24.8 ± 4.3 vs 8.1 ± 2.7 or 8.7 ± 2.3 for the composite lungs for VMAT and IMPT_R, respectively.
Conclusion
The data from this in-silico study suggest that intensity-modulated proton therapy could be significantly advantageous in the treatment of thymoma patients with particular emphasis to a substantial reduction of the risk of cardiac failure and secondary cancer induction. Robust planning is a technical pre-requisite for the safety of the delivery.


Similar content being viewed by others
References
AA.VV (2010) Prescribing recording and reporting photon beam intensity modulated radiation therapy (IMRT), vol 10, issue 1. ICRU Report 83
Ahmad U, Yao X, Detterbeck F, Huang J, Antonicalli A, Filosso P et al (2015) Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 149:95–100
Björk-Eriksson T, Bjelkengren G, Glimelius B (2005) The potentials of proton beam radiation therapy in malignant lymphoma, thymoma and sarcoma. Acta Oncol 44:913–917
Darby S, Ewertz M, McGale P, Bennet A, Blom-Goldman U, Brønnum D et al (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:987–998
Detterbeck F, Youssed S, Ruffini E, Okumura M (2011) A review of prognostic factors in thymic malignancies. J Thorac Oncol 6:S1698–S1704
Engels A (2010) Epidemiology of thymoma and associated malignancies. J Thorac Oncol 5:S260–S265
Feng M, Moran JM, Koelling T et al (2011) Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 79:10–18
Fracchiolla F, Dionisi F, Giacomelli I, Hild S, Esposito P, Lorenzini S et al (2019) Implementation of proton therapy treatments with pencil beam scanning of targets with limited intrafraction motion. Phys Med 57:215–220
Giannopoulou A, Gkiozos I, Harrington K, Syrigos K (2013) Thymoma and radiation therapy: a systematic review of medical treatment. Expert Rev Anticancer Ther 13:759–766
Girard N, Mornex F (2011) The role of radiotherapy in the management of thymic tumors. Thorac Surg Clin 21:99–105
Girard N, Ruffini E, Marz A, Faivre-Finn C, Peters S (2015) ESMO guidelines committee. Thymic epithelial tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v40–v55
Gomez D, Komaki R (2010) Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol 5:S336–S343
Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A (2011) Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol 6:S1743–S1748
Haefner M, Verma V, Bougatf N, Mielke T, Tonndorf-Martini E, König L et al (2018) Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol 57:1713–1720
Hishida T, Nomura S, Yano M, Asamura H, Yamashita M et al (2016) Long term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese nationwide database study. Eur J Cardiothorac Surg 49:835–841
Imbimbo M, Ottaviano M, Vitali M, Fabbri A, Leuzzi G, Fiore M et al (2018) Best practices for the management of thymic epithelial tumors: a position paper by the Italian collaborative group for thymic malignancies (TYME). Cancer Treat Rev 71:76–87
Jackson M, Palma D, Camidge D, Jones B, Robin T, Sher D et al (2017) The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol 12:734–744
Jones B (2017) Proton radiobiology and its clinical implications. Ecancer 11:777
Komaki R, Gomez D (2014) Radiotherapy for thymic carcinoma: adjuvant, inductive, and definitive. Front Oncol 3:330
Korst R, Kansler A, Christos P, Mandal S (2009) Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta analysis. Ann Thorac Surg 87:1641–1647
Kumar V, Gark M, Goyal A, Chaudhari N, Soni P et al (2018) Changing pattern of secondary cancers among patients with malignant thymoma in the USA. Future Oncol 14:1943–1951
Levis M, Filippi A, Fiandra C, De Luca V, Bartoncini S, Vella D et al (2019) Inclusion of heart substructures in the optimization process of volumetric modulated arc therapy techniques may reduce the risk of heart disease in Hodgkin’s lymphoma patients. Radiother Oncol 138:52–58
Li Y, Niemela P, Liao L, Jiang S, Li H, Poenish F et al (2015) Selective robust optimization: a new intensity-modulated proton therapy optimization strategy. Med Phys 42:4840–4847
Liao J, Liu T, Zhang H, Cai F, Chen J, Dang J (2018) The role of postoperative radiation therapy for completely resected stage III thymoma and effect of higher heart radiation dose on risk of cardiovascular disease: a retrospective cohort study. Int J Surg 53:345–349
Lim Y, Kim E, Kim H, Wu H, Yan J, Liu Q, Patel S (2016) Survival impact of adjuvant radiation therapy in Masaoka stage II to IV thymomas: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 94:1129–1136
Liu W, Zhang X, Li Y, Mohan R (2012) Robust optimization of intensity modulated proton therapy. Med Phys 39:1079–1091
Maggi G, Casadio C, Cavallo A, Cianci R, Molinatti M, Ruffini E (1991) Thymoma: results of 241 operated cases. Ann Thorac Surg 51:152–156
McNamara A, Willers H, Paganeti H (2020) Modelling variable proton relative biological effectiveness for treatment planning. Br J Radiol 93:20190334
Mercado C, Hartsell W, Simone C, Tsai H, Vargas C, Zhu H et al (2019) Proton therapy for thymic malignancies: multi-institutional patterns-of-care and early clinical outcomes from the proton collaborative group and the University of Florida prospective registries. Acta Oncol 58:1036–1040
Pan C, Chen P, Wang L, Chi K, Chiang H (2001) Thymoma is associated with an increased risk of second malignancy. Cancer 92:2406–2411
Parikh R, Rhome R, Hug E, Tsai H, Cahlon O, Chon B et al (2016) Adjuvant proton beam therapy in the management of thymoma: a dosimetric comparison and acute toxicities. Clin Lung Cancer 17:362–366
Preston D, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M et al (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
Ruffini E, Mancuso M, Oliaro A, Casadio C, Cavallo A, Cianci R et al (1997) Recurrence of thymoma: analysis of clinicopathologic features, treatment and outcome. J Thorac Cardiovasc Surg 113:55–63
Schneider U, Sumila M, Robotka J (2011a) Site-specific dose–response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Mod 8:27
Schneider U, Sumila M, Robotka J, Gruber G, Mack A, Besserer J (2011b) Dose–response relationship for breast cancer induction at radiotherapy dose. Radiat Oncol 6:67
Siesling S, van de Zwan J, Izarzugaza I, Jaal J, Treasure T et al (2012) Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer 48:949–960
Song Z, Zhang Y (2014) Outcomes after surgical resection of thymic carcinoma: a study from a single tertiary referral center. Eur J Rug Oncol 40:1523–1527
Travis L, Boice J, Travis W (2003) Second primary cancers after thymoma. Int J Cancer 107:869–870
van Nimwegen F, Schaapveld M, Cutter D, Janus C, Krol A, Hauptmann M et al (2016) Radiation dose–response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 34:235–243
van Nimwegen F, Ntentas G, Darby S, Schaapveld M, Hauptmann M, Lugtenburg P et al (2018) Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 129:2257–2265
Vogel J, Berman A, Lin L, Pechet T, Levin W, Gabriel P et al (2016) Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: early response and toxicity assessment. Radiother Oncol 118:504–509
Vogel J, Lin L, Litzky L, Berman A, Simone C (2017) Predicted rate of secondary malignancies following adjuvant proton versus photon radiation therapy for thymoma. Int J Radiat Oncol Biol Phys 99:427–433
Willmann J, Rimner A (2018) The expanding role of radiation therapy for thymic malignancies. J Thorac Dis 10:S2555–2564
Zhou D, Deng X, Liu Q, Zheng H, Min J, Dai J (2016) The effectiveness of postoperative radiotherapy in patients with completely resected thymoma: a meta-analysis. Ann Thorac Surg 101:305–310
Zhu H, Hoppe B, Flampouri S, Louis D, Pirris J, Nichols R et al (2018) Rationale and early outcomes for the management of thymoma with proton therapy. Transl Lung Cancer Res 7:106–113
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Cozzi acts as Scientific Advisor to Varian Medical Systems and is Clinical Research Scientist at Humanitas Cancer Center. All other co-authors declare that they have no conflict interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Humanitas research hospital ethical committee approved by notification this retrospective study (study 10.20).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franceschini, D., Cozzi, L., Loi, M. et al. Volumetric modulated arc therapy versus intensity-modulated proton therapy in the postoperative irradiation of thymoma. J Cancer Res Clin Oncol 146, 2267–2276 (2020). https://doi.org/10.1007/s00432-020-03281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03281-z