Abstract
Background
Iron overload (IOL) due to repetitive transfusions of packed red blood cells (pRBC) has a major impact on morbidity and mortality in patients with inherited bone marrow failure syndromes and hemoglobinopathies such as thalassemia and sickle cell disease. However, whether IOL influences the outcome of elderly patients with myeloid malignancies is not yet clear. Moreover, clinical trials have reported high drop-out rates during treatment with the oral iron chelator deferasirox (DFX).
Aim
Here we report the results of a 2-year prospective observational study that aimed at describing the routine use of DFX in patients with hematological malignancies with regard to safety, efficacy and handling of the drug in a routine setting.
Results
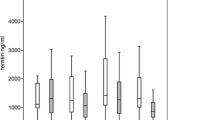
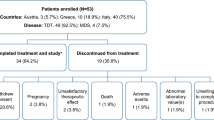
A total of 406 patients were included. 58% of the patients were male. Most of the patients had myelodysplastic syndromes (MDS) (68%) and myeloproliferative neoplasms (MPN) (14%). Median time from first transfusion to study enrollment was 1.1 years (0–25.5 years) and most patients were chelation naive (91%) at enrollment. With regard to transfusion burden, most of the patients were moderately or mildly transfusion-dependent with 53% receiving 2–4 and 27% receiving less than 2 units of pRBC per month. Serum ferritin decreased from a mean of 2305 μg/l (± 1449 μg/l) to a mean of 1910 μg/l (± 1529 μg/l) at 24 months. There was no substantial change in transfusion-dependence during the observation period. Dose adjustments were reported in 48% of the patients with dose-escalation strategies being the most frequent reason for dosage increases (49%). The median observation time was 355 days (5–1080 days). Median duration of exposure to DFX was 322 days (2–1078 days). Two-hundred and ninety (72%) patients discontinued the trial prematurely after a median time of 235 days (1–808 days). Death (29%) and adverse events (23%) were the main reasons for discontinuation. Eleven percent of the patients discontinued treatment due to sufficient decrease in serum ferritin. Most frequent adverse events were decrease in creatinine clearance (22%), increase in serum creatinine (18%) and diarrhea (16%).
Conclusion
This descriptive trial confirms the efficacy of DFX in decreasing the serum ferritin. Moreover, the high drop-out rates seen in prospective trials are recapitulated in this study, which can be attributed to adverse events in a substantial proportion of patients.

Similar content being viewed by others
References
Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC et al (2004) Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89(10):1187–1193
Delforge M, Selleslag D, Beguin Y, Triffet A, Mineur P, Theunissen K et al (2014) Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res 38(5):557–563
Gattermann N, Rachmilewitz EA (2011) Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol 90(1):1–10
Gattermann N, Finelli C, Porta MD, Fenaux P, Ganser A, Guerci-Bresler A et al (2010) Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res 34(9):1143–1150
Kim IH, Moon JH, Lim SN, Sohn SK, Kim HG, Lee GW et al (2015) Efficacy and safety of deferasirox estimated by serum ferritin and labile plasma iron levels in patients with aplastic anemia, myelodysplastic syndrome, or acute myeloid leukemia with transfusional iron overload. Transfusion 55(7):1613–1620
Komrokji RS, Ali NHA, Padron E, Lancet JE, List AF (2011) Impact of iron chelation therapy on overall survival and AML transformation in lower risk MDS patients treated at the moffitt cancer center. Blood 118(21):2776
Latagliata R, Montagna C, Porrini R, Di Veroli A, Leonetti SC, Niscola P et al (2016) Chelation efficacy and erythroid response during deferasirox treatment in patients with myeloproliferative neoplasms in fibrotic phase. Eur J Haematol 96(6):643–649
Lee JW, Yoon SS, Shen ZX, Ganser A, Hsu HC, Habr D et al (2010) Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood 116(14):2448–2454
Leitch HALC., Goodman TA et al (2008) Improved survival in patients with myelodysplastic syndrome receiving iron chelation therapy. Clin Leuk 2(3):205–211
Leitch HA, Parmar A, Wells RA, Chodirker L, Zhu N, Nevill TJ et al (2017) Overall survival in lower IPSS risk MDS by receipt of iron chelation therapy, adjusting for patient-related factors and measuring from time of first red blood cell transfusion dependence: an MDS-CAN analysis. Br J Haematol 179(1):83–97
List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N et al (2012) Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol 30(17):2134–2139
Modell B, Khan M, Darlison M (2000) Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 355(9220):2051–2052
Neukirchen J, Fox F, Kundgen A, Nachtkamp K, Strupp C, Haas R et al (2012) Improved survival in MDS patients receiving iron chelation therapy—a matched pair analysis of 188 patients from the Dusseldorf MDS registry. Leuk Res 36(8):1067–1070
Nolte F, Hochsmann B, Giagounidis A, Lubbert M, Platzbecker U, Haase D et al (2013) Results from a 1-year, open-label, single arm, multi-center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int-1 risk myelodysplastic syndrome (MDS) and transfusion-dependent iron overload. Ann Hematol 92(2):191–198
Rose C, Brechignac S, Vassilief D, Pascal L, Stamatoullas A, Guerci A et al (2010) Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res 34(7):864–870
Taher AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A et al (2017) New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study. Am J Hematol 92(5):420–428
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FN has received research funding and honoraria from Novartis Pharma GmbH; HN, BS, TG, OR, HH, AJ and CS declare no conflict of interest; SA, CJ are employees of Novartis Pharma GmbH; WKH has received research funding from Novartis Pharma GmbH.
Ethical approval
All procedures performed in the trial involving human participants were in accordance with ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nolte, F., Nückel, H., Schmidt, B. et al. Tolerability and efficacy of deferasirox in patients with transfusional iron overload: results from a German 2-year non-interventional study. J Cancer Res Clin Oncol 144, 1531–1538 (2018). https://doi.org/10.1007/s00432-018-2665-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2665-x