Abstract
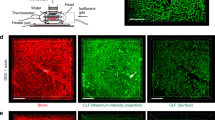
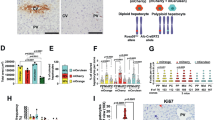
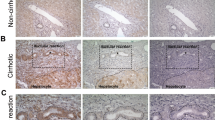
The liver has multiple regeneration modes, including hepatocellular hypertrophy and self-renewal of hepatocytes. When hepatocyte proliferation is impaired, hepatic progenitor cells may proliferate through ductular reaction (DR), differentiate into hepatocytes, and contribute to fibrosis. However, the three-dimensional spatial relationship between DR and regenerating hepatocytes and dynamic changes in DR associated with fibrosis remain poorly understood. Here, we performed three-dimensional (3D) imaging of cleared 42 liver explants with chronic and acute liver diseases and 4 normal livers to visualize DR. In chronic hepatic liver diseases, such as viral hepatitis, steatohepatitis, autoimmune hepatitis, and cryptogenic cirrhosis, the total length and number of branches of DR showed a significant positive correlation. We studied the spatial relationship between DR and GS-expressing cells using glutamine synthetase (GS) and cytokeratin 19 (CK19) as markers of liver regeneration and DR, respectively. The percentage of CK19-positive cells that co-expressed GS was less than 10% in chronic liver diseases. In contrast, nearly one-third of CK19-positive cells co-expressed GS in acute liver diseases, and chronic cholestatic liver diseases, including primary biliary cholangitis and primary sclerosing cholangitis, showed no co-expression. We also found that DR was longer and had more branching in livers with progressive fibrosis compared to those with regressive fibrosis. Our results suggest that DR displays varying degrees of spatial complexity and contribution to liver regeneration. DR may serve as hepatobiliary junctions that maintain continuity between hepatocytes and bile ducts rather than hepatocyte regeneration in chronic liver diseases.





Similar content being viewed by others
Data Availability
All data are available from the corresponding author upon reasonable requests.
Abbreviations
- DR:
-
Ductular reaction
- 3D:
-
Three-dimensional
- 2D:
-
Two-dimensional
- GS:
-
Glutamine synthetase
- CK:
-
Cytokeratin
- DMSO:
-
Dimethyl sulfoxide
- PBS:
-
Phosphate-buffered saline
- DBE:
-
Dibenzyl ether
- PSC:
-
Primary sclerosing cholangitis
- PBC:
-
Primary biliary cholangitis
References
Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P et al (2004) Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39:1739–1745. https://doi.org/10.1002/hep.20130
Popper H, Kent G, Stein R (1957) Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp NY 24:551–556
Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L et al (1999) The canals of hering and hepatic stem cells in humans. Hepatology 30:1425–1433. https://doi.org/10.1002/hep.510300614
Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS et al (2007) Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133:80–90. https://doi.org/10.1053/j.gastro.2007.05.012
Gouw AS, Clouston AD, Theise ND (2011) Ductular reactions in human liver: Diversity at the interface. Hepatology 54:1853–1863. https://doi.org/10.1002/hep.24613
Choi TY, Ninov N, Stainier DY, Shin D (2019) Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146:776–788. https://doi.org/10.1053/j.gastro.2013.10.019
He J, Lu H, Zou Q, Luo L (2013) Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146:789-800.e8. https://doi.org/10.1053/j.gastro.2013.11.045
Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T et al (2015) Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol 17:971–983. https://doi.org/10.1038/ncb3203
Factor VM, Radaeva SA, Thorgeirsson SS (1994) Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathology 145:409–422
Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN et al (2011) Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 53:964–973. https://doi.org/10.1002/hep.24122
Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB et al (2003) Regeneration of hepatocyte “buds” in cirrhosis from intrabiliary stem cells. J Hepatol 39:357–364. https://doi.org/10.1016/s0168-8278(03)00309-x
Stueck AE, Wanless IR (2015) Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology 61:1696–1707. https://doi.org/10.1002/hep.27706
Dezső K, Nagy P, Paku S (2020) Human liver regeneration following massive hepatic necrosis: Two distinct patterns. J Gastroenterol Hepatol 35:124–134. https://doi.org/10.1111/jgh.14721
Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H et al (2014) Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 15:340–349. https://doi.org/10.1016/j.stem.2014.06.003
Schaub JR, Malato Y, Gormond C, Willenbring H (2014) Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep 8:933–939. https://doi.org/10.1016/j.celrep.2014.07.003
Noë M, Rezaee N, Asrani K, Skaro M, Groot VP, Wu PH et al (2018) Immunolabeling of cleared human pancreata provides insights into three-dimensional pancreatic anatomy and pathology. Am J Pathol 188:1530–1535. https://doi.org/10.1016/j.ajpath.2018.04.002
Hong SM, Jung D, Kiemen A, Gaida MM, Yoshizawa T, Braxton AM et al (2020) Three-dimensional visualization of cleared human pancreas cancer reveals that sustained epithelial-to-mesenchymal transition is not required for venous invasion. Mod Pathol 33:639–647. https://doi.org/10.1038/s41379-019-0409-3
Yoshizawa T, Hong SM, Jung D, Noë M, Kiemen A, Wu PH et al (2020) Three-dimensional analysis of extrahepatic cholangiocarcinoma and tumor budding. J Pathol 251:400–410. https://doi.org/10.1002/path.5474
Tainaka K, Kubota SI, Suyama TQ, Susaki EA, Perrin D, Ukai-Tadenuma M et al (2014) Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159:911–924. https://doi.org/10.1016/j.cell.2014.10.034
Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M et al (2014) iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159:896–910. https://doi.org/10.1016/j.cell.2014.10.010
Theise ND, Jia J, Sun Y, Wee A, You H (2018) Progression and regression of fibrosis in viral hepatitis in the treatment era: the Beijing classification. Mod Pathol 31:1191–1200. https://doi.org/10.1038/s41379-018-0048-0
Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A (2012) Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol 22:1166–1175. https://doi.org/10.1016/j.cub.2012.05.016
Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M (2006) Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 43:817–825. https://doi.org/10.1002/hep.21131
Cadoret A, Ovejero C, Terris B, Souil E, Lévy L, Lamers WH et al (2002) New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 21:8293–8301. https://doi.org/10.1038/sj.onc.1206118
Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T et al (2010) Beta-Catenin Activation Promotes Liver Regeneration after Acetaminophen-Induced Injury. Am J Pathol 175:1056–1065. https://doi.org/10.2353/ajpath.2009.080976
Fleming KE, Wanless IR (2013) Glutamine synthetase expression in activated hepatocyte progenitor cells and loss of hepatocellular expression in congestion and cirrhosis. Liver Int 33:525–534. https://doi.org/10.1111/liv.12099
Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S et al (2012) Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143:1564-1575.e7. https://doi.org/10.1053/j.gastro.2012.08.024
Clerbaux LA, Manco R, Van Hul N, Bouzin C, Sciarra A, Sempoux C et al (2019) Invasive ductular reaction operates hepatobiliary junctions upon hepatocellular injury in rodents and humans. Am J Pathology 189:1569–1581. https://doi.org/10.1016/j.ajpath.2019.04.011
Wanless IR, Nakashima E, Sherman M (2000) Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med 124:1599–1607. https://doi.org/10.5858/2000-124-1599-ROHC
Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR (2005) Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 41:809–818. https://doi.org/10.1002/hep.20650
Izumi T, Imai J, Yamamoto J, Kawana Y, Endo A, Sugawara H et al (2018) Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat Commun 9:5300–5313. https://doi.org/10.1038/s41467-018-07747-0
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N et al (1999) Bone marrow as a potential source of hepatic oval cells. Science 284:1168–1170. https://doi.org/10.1126/science.284.5417.1168
Funding
This study was supported by the Department of Pathology at the John Hopkins University School of Medicine.
Author information
Authors and Affiliations
Contributions
TY and KO designed the study. TY, SH, and DJ performed experiments and data analysis. MN, WZ, AK, PW, and DW provided technical support. JWL, RHH, LDW, and KO performed data analysis and interpreted data. TY and KO developed methods and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Studies were conducted in accordance with the 1996 Declaration of Helsinki and approved by the institutional review board (IRB) at the Johns Hopkins Hospital.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
428_2023_3641_MOESM1_ESM.tif
Supplementary file1 Supplementary Figure 1 |Quantification of unit volume. The nodule diameter (R) was measured (A) and the volume was set as shown in (B) (R/2 × R/4 × R/8). Scale bar represents 100 μm (TIF 9840 KB)
Supplementary file2 Supplementary Movie 1 | 3D surface rendering image of bile ducts in control liver. Bile ducts are highlighted by CK19, which is shown in green. (MP4 113931 KB)
Supplementary file3 Supplementary Movie 2 | 3D surface rendering image of DR and GS in chronic hepatic liver diseases. DR is highlighted by CK19. CK19 and GS are shown in green and red, respectively. Cells co-expressing CK19 and GS are shown in silver. (MP4 212073 KB)
Supplementary file4 Supplementary Movie 3 | 3D surface rendering image of DR and GS in acute liver diseases. DR is highlighted by CK19. CK19 and GS are shown in green and red, respectively. Cells co-expressing CK19 and GS are shown in silver. (MP4 362346 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshizawa, T., Lee, J.W., Hong, SM. et al. Three-dimensional analysis of ductular reactions and their correlation with liver regeneration and fibrosis. Virchows Arch (2023). https://doi.org/10.1007/s00428-023-03641-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-023-03641-3