Abstract
Aims
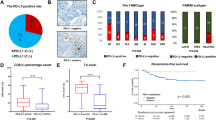
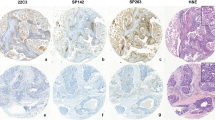
SP142 and 22C3 assays are approved companion diagnostic assays for anti-PD-1/PD-L1 therapy selection in metastatic triple-negative breast cancer (TNBC). The discordance in PD-L1 status between primary and metastatic tumors in the same patient has been poorly characterized. Here, we examined the concordance of PD-L1 status between the two assays and between primary tumors and metastases for each assay.
Methods
We retrospectively evaluated tumor samples from 160 patients with TNBC, including 45 patients with paired primary and metastatic tumors. PD-L1 status was assessed using SP142 and 22C3 assays, to determine the immune cell (IC) score, tumor cell (TC) score (SP142 and 22C3), and combined proportion score (CPS: 22C3).
Results
The concordance of PD-L1 positivity at diagnostic cutoffs for SP142 (IC ≥ 1) and 22C3 (CPS ≥ 10) was substantial (κ = 0.80) in primary tumors and moderate (κ = 0.60) in metastatic tumors. In comparison, between primary and metastatic tumors, the concordance with 22C3 was moderate (κ = 0.50), whereas that with SP142 was poor (κ = -0.03). Among patients who were PD-L1 negative for both assays in primary tumors, 7/30 (23.3%) were PD-L1 positive for both or either 22C3 or SP142 in the metastatic tumors.
Conclusions
The inter-assay concordance of PD-L1 positivity at diagnostic cutoffs was substantial in primary tumors and moderate in metastatic tumors. Discordance between PD-L1 status in primary and metastatic tumors was frequently observed, especially with SP142. Some patients with a PD-L1-negative status in primary tumors may still be candidates for immunotherapy, depending on the PD-L1 status in their metastatic tumors.



Similar content being viewed by others
Data availability statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Howard FM, Olopade OI (2021) Epidemiology of triple-negative breast cancer: a review. Cancer J 27(1):8–16. https://doi.org/10.1097/PPO.0000000000000500
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt. 1):4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045
Schmid P, Rugo HS, Adams S et al (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21(1):44–59. https://doi.org/10.1016/S1470-2045(19)30689-8
Cortes J, Cescon DW, Rugo HS et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Hoda RS, Brogi E, Dos Anjos CH et al (2020) Clinical and pathologic features associated with PD-L1 (SP142) expression in stromal tumor-infiltrating immune cells of triple-negative breast carcinoma. Mod Pathol 33(11):2221
Yazaki S, Salgado R, Shimoi T et al (2023) Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer. Br J Cancer 128(4):568–575. https://doi.org/10.1038/s41416-022-02072-2
Lee SE, Park HY, Lim SD et al (2020) Concordance of programmed death-ligand 1 expression between SP142 and 22C3/SP263 assays in triple-negative breast cancer. J Breast Cancer 23(3):303–313. https://doi.org/10.4048/jbc.2020.23.e37
Emens LA, Molinero L, Loi S et al (2021) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 Study. J Natl Cancer Inst 113(8):1005–1016. https://doi.org/10.1093/jnci/djab004
Loi S, Salgado R, Schmid P et al (2023) Association between biomarkers and clinical outcomes of pembrolizumab monotherapy in patients with metastatic triple-negative breast cancer: KEYNOTE-086 exploratory analysis. JCO Precis Oncol 7:e2200317. https://doi.org/10.1200/PO.22.00317
Gonzalez-Ericsson PI, Stovgaard ES, Sua LF et al (2020) The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J Pathol 250(5):667–684. https://doi.org/10.1002/path.5406
Yazaki S, Shimoi T, Yoshida M et al (2023) Integrative prognostic analysis of tumor-infiltrating lymphocytes, CD8, CD20, programmed cell death-ligand 1, and tertiary lymphoid structures in patients with early-stage triple-negative breast cancer who did not receive adjuvant chemotherapy. Breast Cancer Res Treat 197(2):287–297. https://doi.org/10.1007/s10549-022-06787-x
Villegas SL, Nekljudova V, Pfarr N et al (2021) Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors – An analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer 148:159–170. https://doi.org/10.1016/j.ejca.2021.02.020
Schrodi S, Braun M, Andrulat A et al (2021) Outcome of breast cancer patients with low hormone receptor positivity: analysis of a 15-year population-based cohort. Ann Oncol 32(11):1410–1424. https://doi.org/10.1016/j.annonc.2021.08.1988
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (Poplar): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Lawson NL, Dix CI, Scorer PW et al (2020) Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod Pathol 33(4):518–530. https://doi.org/10.1038/s41379-019-0372-z
Shafi S, Parwani AV, Li Z (2022) PD-L1 (SP142 and 22C3) immunohistochemistry in clinical metastatic triple-negative or low hormone receptor breast carcinomas: experience from a large academic institution. Hum Pathol 126:100–107. https://doi.org/10.1016/j.humpath.2022.05.013
Hirsch FR, McElhinny A, Stanforth D et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from Phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12(2):208–222. https://doi.org/10.1016/j.jtho.2016.11.2228
Tsao MS, Kerr KM, Kockx M et al (2018) PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint Phase 2 project. J Thorac Oncol 13(9):1302–1311. https://doi.org/10.1016/j.jtho.2018.05.013
Ahn S, Woo JW, Kim H et al (2021) Programmed death ligand 1 immunohistochemistry in triple-negative breast cancer: evaluation of inter-pathologist concordance and inter-assay variability. J Breast Cancer 24(3):266
Rugo HS, Loi S, Adams S et al (2021) PD-L1 immunohistochemistry assay comparison in atezolizumab plus nab-paclitaxel–treated advanced triple-negative breast cancer. J Natl Cancer Inst 113(12):1733–1743. https://doi.org/10.1093/jnci/djab108
Huang X, Ding Q, Guo H et al (2021) Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum Pathol 108:42–50. https://doi.org/10.1016/j.humpath.2020.11.004
Stovgaard ES, Bokharaey M, List-Jensen K et al (2020) PD-L1 diagnostics in the neoadjuvant setting: implications of intratumoral heterogeneity of PD-L1 expression in triple negative breast cancer for assessment in small biopsies. Breast Cancer Res Treat 181(3):553–560. https://doi.org/10.1007/s10549-020-05655-w
Kalpakoff M, Hund S, Musser J et al (2021) Intrapatient tumor heterogeneity in IHC interpretation using PD-L1 IHC 22C3 pharmDx. Appl Immunohistochem Mol Morphol 29(9):667–673. https://doi.org/10.1097/PAI.0000000000000941
Acknowledgements
We thank Shoichi Harada, Sachiko Miura, Toshiko Sakaguchi, and Chizu Kina for technical assistance. We thank Editage (http://www.editage.com) for English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JM, SY, and MY made substantial contributions to the study concept, the data analysis or interpretation, and drafting of the original manuscript; SY, TS, MO, AS, SK, KY, YK, HS(O), TN, KS, EN, TM, SS, ST, AS, YF, and KY made substantial contributions to the acquisition of data in this study; All authors reviewed the manuscript draft, revised it critically on intellectual content, and approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Ethics approval statement
This study was approved by the Institutional Review Board of the National Cancer Center (No. 2014–092).
Competing isnterests
Masayuki Yoshida reports personal fees from Roche Japan, Eli Lilly, Chugai, MSD, Daiichi Sankyo, Ono, Agilent technologies outside of the submitted work. Tadaaki Nishikawa reports personal fees from Takeda Pharmaceutical Company, Eisai, and AstraZeneca outside of the submitted work. Emi Noguchi reports personal fees from Pfizer, Taiho, Eli Lilly, AstraZeneca, Chugai, and Eisai outside the submitted work. Kan Yonemori reports receiving grants from Merck Sharp & Dohme, Daiichi-Sankyo, AstraZeneca, Taiho, Pfizer, Novartis, Takeda, Chugai, Ono, Seattle Genetics, Eisai, Eli Lilly, Genmab, Boehringer Ingelheim, Kyowa Hakko Kirin, Nihon Kayaku, and Haihe; personal fees from Pfizer, Eisai, Astrazeneca, Eli Lilly, Takeda, Chugai, MSD, FujiFilm Pharma, Bayer, Asteras, Boeringer Ingelheim, Daiichi Sankyo, PDR pharma, Sanofi outside the submitted work.
Patient consent statement
The need for informed consent was waived because of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miyakoshi, J., Yazaki, S., Shimoi, T. et al. Discordance in PD-L1 expression using 22C3 and SP142 assays between primary and metastatic triple-negative breast cancer. Virchows Arch 483, 855–863 (2023). https://doi.org/10.1007/s00428-023-03634-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03634-2