Abstract
Male breast cancer (MBC) is a rare disease. Due to its rarity, treatment is still directed by data mainly extrapolated from female breast cancer (FBC) treatment, despite the fact that it has recently become clear that MBC has its own molecular characteristics. DDX3 is a RNA helicase with tumor suppressor and oncogenic potential that was described as a prognosticator in FBC and can be targeted by small molecule inhibitors of DDX3. The aim of this study was to evaluate if DDX3 is a useful prognosticator for MBC patients. Nuclear as well as cytoplasmic DDX3 expression was studied by immunohistochemistry in a Dutch retrospective cohort of 106 MBC patients. Differences in 10-year survival by DDX3 expression were analyzed using log-rank test. The association between clinicopathologic variables, DDX3 expression, and survival was tested in uni- and multivariate Cox-regression analysis. High cytoplasmic DDX3 was associated with high androgen receptor (AR) expression while low nuclear DDX3 was associated with negative lymph node status. Nuclear and cytoplasmic DDX3 were not associated with each other. In a univariate analysis, high cytoplasmic DDX3 (p = 0.045) was significantly associated with better 10-year overall survival. In multivariate analyses, cytoplasmic DDX3 had independent prognostic value (p = 0.017). In conclusion, cytoplasmic DDX3 expression seems to be a useful prognosticator in MBC, as high cytoplasmic DDX3 indicated better 10-year survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male breast cancer (MBC) is a rare disease. Less than 1% of all male cancer patients have breast cancer and males account for less than 1% of all breast cancers [1]. Due to its rarity, the treatment and prediction of MBC is still directed by data that are mainly extrapolated from randomized prospective studies or clinical experience of female breast cancer (FBC) treatment, despite the fact that during the last decade more and more has become known about the unique tumor biology of MBC [1,2,3,4,5].Nowadays, the focus in oncology is to prevent overtreatment and gain quality of life while maintaining survival rates. The average age of male patients diagnosed with breast cancer is around 65 years, compared to 55 for female patients [4, 6,7,8]. Overtreatment with adjuvant chemotherapy should be avoided because of the side effects, especially at older age. Moreover, the side effects of hormonal therapy in males are significant. If enhanced survival can be predicted, better decisions can be made for personalized chemo- and hormonal therapy.
DDX3, a member of the RNA helicase family, has multiple functions in a variety of cellular biogenesis processes [9], including cell-cycle regulation, [10], translation regulation [11, 12], DNA repair, cell survival and apoptosis [13]. DDX3 can shuffle between nucleus and cytoplasm. Cytoplasmic DDX3 and nuclear DDX3 can be measured separately. The various, often opposing roles of DDX3, both nuclear and cytoplasmic [10, 14] have been studied in several cancer types. DDX3 was reported as an oncogene in Ewing sarcoma, breast cancer [15, 16] prostate cancer [14], gallbladder carcinoma as well as pancreatic ductal adenocarcinoma [17], while being tumor suppressive in melanoma [18]. Meanwhile, for lung cancer [12], colorectal cancer [19, 20], hepatocellular carcinoma [21] and oral squamous cell carcinoma [22], literature is not uniform with regard to the role of DDX3; both oncogenic as well as tumor suppressive roles have been described [20]. If DDX3 overexpression would be relevant in MBC, this could create therapeutic options because several publications have shown that DDX3 can be targeted by the small molecule RK-33 [23,24,25]. Nuclear DDX3 was previously described as a negative prognostic factor in FBC [25, 26], while cytoplasmic over-expression of DDX3 was found in FBC brain metastases of especially triple negative and high grade cases [16], but the prognostic value of DDX3 in MBC had not yet been studied. The aim of this study was therefore to evaluate if DDX3 might be a useful prognosticator for MBC-patients.
Material and methods
Patient samples
All consecutive cases of surgical breast specimens of invasive MBC from 1986–2011 were collected from five different pathology laboratories in The Netherlands: St Antonius Hospital Nieuwegein (n = 41), Diakonessenhuis Utrecht (n = 34), University Medical Center Utrecht (n = 29), Gelre hospital Apeldoorn (n = 17) and Laboratory for Pathology East Netherlands (n = 40). Pathology reports were used to extract age, tumor size, and lymph node status. Cases with isolated tumor cells in the sentinel node were coded as lymph node negative. Grade, according to the modified Bloom and Richardson score [27], Mitotic Activity Index (MAI), histologic subtype, Ki67 (low < = 10), androgen (AR), estrogen (ER; positive > = 1%) [28] and progesterone receptor (PR; positive > = 10%) and human epidermal growth factor receptor 2 (HER2) status were obtained from previous studies [2, 3, 29, 30]. The MBC tissue blocks were gathered and tissue arrays were constructed as described before [2]. In short, hematoxylin and eosin stained slides were used to identify representative tumor areas. From these areas three 0.6-mm punch biopsies from formalin-fixed and paraffin-embedded tissue blocks were obtained and embedded in a recipient paraffin block, using a precision tissue array instrument (Beecher Instruments).
Follow-up data were obtained anonymously through the Comprehensive Cancer Center of The Netherlands (IKNL) as well as by retrieving data from the Dutch central pathology administration system PALGA. The most recent date of information was used as date of last follow-up. Recurrence was coded positive if described in the follow-up data and/or proven by pathologic investigation. Overall survival (OS) was defined as the interval from surgery to death from any cause or date of lost to follow-up. For the latter, the date of the latest pathology report was used, which could concern recurrence or distant metastasis or death or benign pathologic findings. It was possible to analyze DDX3 in 106 patients of whom follow-up data were available. For this study only anonymous archival leftover pathology material was used. Therefore no informed consent was required according to Dutch legislation, which uses an opt-out system.
Immunohistochemistry
As before [16], four µm thick sections were cut, mounted on Surgipath X-tra adhesive slides (Leica Biosystems, Milton Keynes, UK), deparaffinized in xylene and rehydrated in decreasing ethanol dilutions. Endogenous peroxidase activity was blocked with 1.5% hydrogen peroxide buffer for 15 min, followed by antigen retrieval by boiling for 20 min in EDTA buffer (pH 9.0). Slides were blocked with protein block from Novolink Polymer Detection System (Leica Microsystems, Eindhoven, The Netherlands) and subsequently incubated in a humidified chamber for 1 h with anti-DDX3 (1:50, mAb AO196) [31]. Post primary block, secondary antibodies and diaminobenzidine treatment were performed with the same Novolink Polymer Detection System according to the manufacturer’s instructions. The slides were lightly counterstained with hematoxylin and mounted. Appropriate positive and negative controls were used throughout. Nuclear and cytoplasmic DDX3 staining was scored by consensus of two observers (PvD and CvdP) and interpreted according to methods described earlier [16, 23, 32,33,34]. In short, the percentage of DDX3-positive nuclei was scored. Samples with ≥ 1% DDX3 staining were regarded positive. Cytoplasmic DDX3 was scored semiquantitatively as absent (0), low (1), moderate (2) or strong (3). Cases with score 0–2 were classified as having low DDX3 expression and evaluated against cases with strong expression. For comparison, DDX3 expression data of ER + /HER2- female breast cancer patients were taken from our earlier research [25, 35].
Statistics
Statistical analyses were performed with SPSS IBM Statistics version 25. To assess the association between clinicopathological variables and DDX3 expression in MBC patients, and to compare MBC with female breast cancer patients, Pearson Chi-square- and Fisher’s exact tests were performed. For survival analysis, Kaplan–Meier curves were plotted with stratification for DDX3 expression (high/low). Differences in 10-year survival were compared with the logrank test. Cox uni- and multivariate regression analysis (enter-method) was used to assess the clinicopathological factors as well as DDX3 associated with 10-year survival. The factors that reached statistical significance in univariate analysis were used in multivariate analysis. Significance was defined as p < 0.05.
Results
As shown in Table 1, the median age of the 106 patients 66.5 years. Most men were treated for T1-2 tumors (76.4%) and there were 23 patients with T4 breast cancer (21.7%). The vast majority (93.4%) of tumors were of no special type (formerly “ductal”; 96 ductal, 1 neuro endocrine and 2 ductulolobular) and there were one adenoid cystic, one invasive lobular carcinoma and 5 (4,7%) other invasive types (2 mucinous, 1 inverted papillary, 1 intracystic papillary and 1 cribriform cancer). 92.5% of the patients were ER positive, 64.2% PR positive and 95.3% HER2 negative. AR was noted for 71 patients of whom 34 were positive (48%). Defining breast cancer intrinsic subtypes by quantitative receptor expression showed 73.6% (n = 78) luminal A (ER + or PR + , HER2-, MIB1 < 14%), 17.9% (n = 19) luminal B (ER + or PR + , HER2 ± , MIB1 ≥ 14%), 0% HER2 driven and 2.8% (n = 3) basal-like (ER-/PR-/HER2-) cancers while 6 cases could not be defined because of missing data. The AR positive patients were mainly ER + /PR + /HER2- (70.2%) or ER + /PR-/HER2- (26.3%), while only 3.5% ER + /PR-/HER2 + and none ER + /PR + /HER2 + or triple negative.
Lymph node status was mostly N0 (46.2%) or N1 (23.6%). Most patients underwent a radical mastectomy (n = 91), while 5 patients were treated with breast conserving treatment. Twelve patients received adjuvant chemo- and 45 adjuvant antihormonal treatment. No neoadjuvant chemotherapy was administered.
Associations between DDX3 expression and clinicopathologic variables
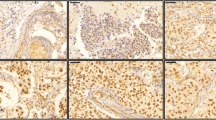
DDX3 expression was seen in the cytoplasm and the nucleus. Figure 1 shows representative examples. In 61 patients cytoplasmic DDX3-expression was low (57.5%) while the majority of cases displayed low nuclear DDX3 (79.2%). As shown in Table 2, low nuclear DDX3 was significantly associated with negative lymph node status and AR positivity was significantly associated with high cytoplasmic DDX3. ER, PR and HER2 status showed no significant associations with nuclear or cytoplasmic DDX3 expression. Nuclear and cytoplasmic DDX3 expression were not mutually exclusive and not significantly associated (Table 3).
Comparison between DDX3 expression in male and female breast cancer
MBC-patients had higher cytoplasmic (p = 0.005) and lower nuclear DDX3 (p = 0.003) levels compared to female breast cancer patients.
Survival analysis
Mean overall survival (OS) was 7 years, with a median of 5.7 years (range 29 days – 25 years). 10-year OS was 60.4% with a median of 5.7 year. In univariate survival analysis, cytoplasmic DDX3 (p = 0.045), age, T- and PR-status were statistically significant prognosticators (Table 4), older age, higher T-stage, negative PR-status and low cytoplasmic DDX3 expression being unfavorable. Figure 2 shows the Kaplan Meier curves for patients with low and high cytoplasmic DDX3. In multivariate analyses, cytoplasmic DDX3 expression appeared to have independent prognostic value for 10 year survival (p = 0.017) (Table 4). Nuclear DDX3 expression had no prognostic value in uni- or multivariate survival analysis.
Discussion
This is the first report on the prognostic value of DDX3 in MBC, indicating that cytoplasmic DDX3 seems to be a useful prognostic biomarker; low expression being associated with a more unfavorable outcome.
The present results are at variance from data on DDX3 expression in FBC, where high nuclear DDX3 in the primary tumor was described as an independent predictor of worse survival [15, 16] and cytoplasmic DDX3 was not. For metastases in FBC, on the contrary, cytoplasmic DDX3 was found to be overexpressed especially for the more aggressive types like the triple negatives and grade 3 [16]. Low nuclear DDX3 in our study was associated with favorable N-status (Table 2), but of no statistically significant influence on survival. In MBC it is plausible that the oncogenic functions of DDX3 are not as evident compared to the tumor suppressive role of DDX3 [25]. Also, DDX3 has two different phenotypes, unstable and stable and the gene can have mutations in some specific sites in some types of cancer [10]. For prostate cancer, different cytoplasmic expression levels were described as well, but no association was found with survival [36].
DDX3 is a DEAD-box-RNA-helicase involved in several biogenesis cell-activities [9,10,11,12,13, 37]. In the literature DDX3 is described to be predominantly localized in the cytoplasm [10]. DDX3 shuttles between cytoplasm and nucleus in most human tissues and cell lines [32]. In the nucleus, DDX3 has roles in transcription, splicing, and nuclear export [10]. Cytoplasmic DDX3 moreover acts as a translation regulator and may influence cell division and/or cell growth [10, 14, 15]. Brennan studied the nucleo-cytoplasmic shuttling of DDX3 and confirmed that an N-terminal conserved Nuclear Export Signal is required for export of human DDX3 from the nucleus. Three regions were identified within DDX3 that can independently facilitate its nuclear import [10]. Different protein-binding-complexes play a role in movement of DDX3 from cytoplasm to nucleus and vice versa [10, 11, 16, 20, 35, 38]. These protein-binding-complexes appear to be different for different tumor types. Association of high nuclear DDX3 and worse prognosis has been attributed to disturbed export of DDX3 from nucleus to cytoplasm, rather than elevated import of DDX3 into the nucleus [10]. It has also been described that DDX3 subcellular localization is cell cycle dependent; more cytoplasmic in G0/1- and S-phase, and more nuclear in G2/M phase [10]. This might be a reasonable explanation for our finding that elevated cytoplasmic DDX3 is associated with a better prognosis: more cells in G0/1- or S-phase matches with most MBC being luminal A and grade 2 [1, 39]. Indeed, almost 70% was N0-N1 (Table 1) and 32/44 cases with high cytoplasmic DDX3 expression in the present study had a low Ki67 index (Table 2).
There is no convincing gender-specific explanation for the difference in prognostic value of DDX3 between MBC- and FBC-patients. Zhao, however, found that the expression level of DDX3 in hepatocellular carcinoma (HCC) was gender related and that the tendency of DDX3 down-regulation in HCC was more frequently found in males than in females [20]. It remains unclear if this concerned nuclear- and/or cytoplasmic DDX3 expression. In human there are 2 types of DDX3; DDX3X and DDX3Y, located on respectively the X- and Y-chromosomes. DDX3X and DDX3Y are 92% homologous. DDX3Y is especially important for spermatogenesis and male fertility [40], but also dual correlation of DDX3Y with cancer patient survival in different cancer types has been described [18]. Whether DDX3Y acts as a functional substitute for the loss of DDX3X in some contexts, remains unclear [41]. Lin suggests that gender differences could be explained by the fact that DDX3, located on the X-chromosome, is preferentially mutated in males [25]. If DDX3 can escape X-inactivation, females may be protected from complete functional loss by a single gene mutation. Besides this, in the same study, a possible explanation of a lower DDX3 level in males was suggested by the fact that DDX3 expression is closely associated with living habits, including smoking, alcohol consumption and other habits which are more frequent in males than in females [17]. The literature on the effect of smoking on DDX3 levels and the association with prognosis, however, is not uniform [22, 42]. Chang described a 1.5–threefold elevated DDX3 for women compared to men in normal liver tissue [21] but not clearly specified on cytoplasmic or nuclear DDX3. Trying to further explain our results, we compared cytoplasmic- and nuclear DDX3 of MBC patients of the current cohort with the ER + /HER2- FBC patients of our earlier research [25, 35], including ER-positive and HER2-negative tumors. Statistically significant gender specific differences were found (p = 0.005); MBC-patients had higher levels of cytoplasmic DDX3 and lower nuclear DDX3 compared to FBC patients.
Androgen receptor expression was statistically significant associated with cytoplasmic DDX3 in this study. High AR in ER-positive FBC patients was associated with a better prognosis in a previous study [43] and thus is in line with our findings. This is an interesting finding as in prostate cancer, especially in castration-resistant prostate cancer, high cytoplasmic DDX3 expression was associated with lower AR expression [44]. Either different DDX3 roles in different types of cancer, oncogenic or suppressive, or the small amount of AR measured MBC patients in this study, could be an explanation for these differences.
Limitations of this study are the wide range in date of diagnosis and treatment between the first and last patient included in this cohort and the relatively small cohort size. However, MBC is a rare disease and our cohort is comparable to the literature; most of the tumors are ER-positive, HER2 negative and Ki67% low and grade 2 [1, 39]. Also consistent with the literature is the first presentation in a more advanced stage; in our cohort 21.7% had a T4 at presentation [6, 7]. Our analysis was further limited by not having full insight into the compliance on adjuvant therapy whereas adherence to antihormonal treatment in men is expected to be low, knowing that for FBC patients non-compliance descending to 50% in 4 year has been described [45].
In conclusion, DDX3 is a multifunctional protein and the regulatory mechanisms and signaling pathways of DDX3 are disease specific. Although the exact mechanism of action of DDX3 in MBC is not clear, cytoplasmic DDX3 expression seems to be a useful prognosticator in MBC, high cytoplasmic DDX3 indicating better 10-year overall survival associated with low proliferation. Thereby, our results rather support a tumor suppressor role of DDX3 in MBC.
References
Cardoso F, Bartlett JMS, Slaets L et al (2018) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann Oncol 29(2):405–417. https://doi.org/10.1093/annonc/mdx651
Kornegoor R, Verschuur-Maes AHJ, Buerger H et al (2012) Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol 25(3):398–404. https://doi.org/10.1038/modpathol.2011.174
Kornegoor R, Verschuur-Maes AHJ, Buerger H et al (2012) Immunophenotyping of male breast cancer. Histopathology 61(6):1145–1155. https://doi.org/10.1111/j.1365-2559.2012.04330.x
Abreu MH, Afonso N, Abreu PH et al (2016) Male breast cancer: looking for better prognostic subgroups. Breast 26:18–24. https://doi.org/10.1016/j.breast.2015.12.001
Fentiman IS (2018) The biology of male breast cancer. Breast 38:132–135. https://doi.org/10.1016/j.breast.2018.01.001
Wang F, Shu X, Meszoely I et al (2019) Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol 5(11):1589–1596. https://doi.org/10.1001/jamaoncol.2019.2803
Miao H, Verkooijen HM, Chia K-S et al (2011) Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol 29(33):4381–4386. https://doi.org/10.1200/JCO.2011.36.8902
Contractor KB, Kaur K, Rodrigues GS et al (2008) Male breast cancer: is the scenario changing. World J SurgOncol 6:58. https://doi.org/10.1186/1477-7819-6-58
Guenther U, Weinberg DE, Zubradt MM et al (2018) The helicase Ded1p controls use of near-cognate translation initiation codons in 5′ UTRs. Nature 559(7712):130–134. https://doi.org/10.1038/s41586-018-0258-0
Brennan R, Haap-Hoff A, Gu L et al (2018) Investigating nucleo-cytoplasmic shuttling of the human DEAD-box helicase DDX3. Eur J Cell Biol 97(7):501–511. https://doi.org/10.1016/j.ejcb.2018.08.001
Chen H-H, Yu H-I, Cho W-C et al (2015) DDX3 modulates cell adhesion and motility and cancer cell metastasis via Rac1-mediated signaling pathway. Oncogene 34(21):2790–2800. https://doi.org/10.1038/onc.2014.190
Bol GM, Xie M, Raman V (2015) DDX3, a potential target for cancer treatment. Mol Cancer 14(1). https://doi.org/10.1186/s12943-015-0461-7
Sun M, Zhou T, Jonasch E et al (2013) DDX3 regulates DNA damage-induced apoptosis and p53 stabilization. BiochimBiophysActa - Mol Cell Res 1833(6):1489–1497. https://doi.org/10.1016/j.bbamcr.2013.02.026
Vellky JE, Ricke EA, Huang W, Ricke WA (2019) Expression and localization of DDX3 in prostate cancer progression and metastasis. Am J Pathol 189(6):1256–1267. https://doi.org/10.1016/j.ajpath.2019.02.011
Botlagunta M, Vesuna F, Mironchik Y et al (2008) Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27(28):3912–3922. https://doi.org/10.1038/onc.2008.33
Heerma van Voss MR, Schrijver WAME, ter Hoeve ND et al (2017) The prognostic effect of DDX3 upregulation in distant breast cancer metastases. Clin Exp Metastasis 34(1):85–92. https://doi.org/10.1007/s10585-016-9832-8
He Y, Zhang D, Yang Y et al (2018) A double-edged function of DDX3, as an oncogene or tumor suppressor, in cancer progression (Review). Oncol Rep 39(3):883–892. https://doi.org/10.3892/or.2018.6203
Lin TC (2020) DDX3X multifunctionally modulates tumor progression and serves as a prognostic indicator to predict cancer outcomes. Int J Mol Sci 21(1). https://doi.org/10.3390/ijms21010281
Botlagunta M, Krishnamachary B, Vesuna F et al (2011) Expression of DDX3 is directly modulated by hypoxia inducible factor-1 alpha in breast epithelial cells. Batra SK, ed. PLoS One 6(3):e17563. https://doi.org/10.1371/journal.pone.0017563
Zhao L, Mao Y, Zhou J et al (2016) Multifunctional DDX3: Dual roles in various cancer development and its related signaling pathways. Am J Cancer Res 6(2):387–402
Chang PC, Chi CW, Chau GY et al (2006) DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene 25(14):1991–2003. https://doi.org/10.1038/sj.onc.1209239
Heerma van Voss MR, van Kempen PMW, Noorlag R et al (2015) DDX3 has divergent roles in head and neck squamous cell carcinomas in smoking versus non-smoking patients. Oral Dis 21(2):270–271. https://doi.org/10.1111/odi.12299
Bol GM, Vesuna F, Xie M et al (2015) Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol Med 7(5):648–669. https://doi.org/10.15252/emmm.201404368
Tantravedi S, Vesuna F, Winnard PT et al (2019) Targeting DDX3 in medulloblastoma using the small molecule inhibitor RK-33. Transl Oncol 12(1):96–105. https://doi.org/10.1016/j.tranon.2018.09.002
Heerma van Voss MR, Vesuna F, Bol GM et al (2018) Targeting mitochondrial translation by inhibiting DDX3: a novel radiosensitization strategy for cancer treatment. Oncogene 37(1):63–74. https://doi.org/10.1038/onc.2017.308
Bol GM, Raman V, van der Groep P et al (2013) Expression of the RNA Helicase DDX3 and the hypoxia response in breast cancer. PLoS ONE 8(5):1–7. https://doi.org/10.1371/journal.pone.0063548
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x
Hammond MEH, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Verschuur-Maes AHJ, Kornegoor R, de Bruin PC et al (2014) Do columnar cell lesions exist in the male breast? Histopathology 64(6):818–825. https://doi.org/10.1111/his.12333
Lacle MM, van der Pol C, Witkamp A et al (2013) Prognostic value of mitotic index and Bcl2 expression in male breast cancer. PLoS ONE 8(4):e60138. https://doi.org/10.1371/journal.pone.0060138
Angus AG, Dalrymple D, Boulant S et al (2010) Requirement of cellular DDX3 for hepatitis C virus replication is unrelated to its interaction with the viral core protein. J Gen Virol 91(1):122–132. https://doi.org/10.1099/vir.0.015909-0
Yedavalli VSRK, Neuveut C, Chi YH et al (2004) Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119(3):381–392. https://doi.org/10.1016/j.cell.2004.09.029
Budczies J, Klauschen F, Sinn BV et al (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 7(12):1–7. https://doi.org/10.1371/journal.pone.0051862
Heerma-van-Voss MR, Vesuna F, Trumpi K et al (2015) Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget 6(29):28312–28326. https://doi.org/10.18632/oncotarget.4873
Heerma-Van-Voss MR, Vesuna F, Bol GM et al (2017) Nuclear DDX3 expression predicts poor outcome in colorectal and breast cancer. Onco Targets Ther 10:3501–3513. https://doi.org/10.2147/OTT.S140639
Xie M, Vesuna F, Tantravedi S et al (2016) RK-33 Radiosensitizes Prostate cancer cells by blocking the RNA helicase DDX3. Cancer Res 76(21):6340–6350. https://doi.org/10.1158/0008-5472.CAN-16-0440
Chen WJ, Wang WT, Tsai TY et al (2017) DDX3 localizes to the centrosome and prevents multipolar mitosis by epigenetically and translationally modulating p53 expression. Sci Rep 7(1):1–20. https://doi.org/10.1038/s41598-017-09779-w
Botlagunta M, Krishnamachary B, Vesuna F et al (2011) Expression of DDX3 is directly modulated by hypoxia inducible factor-1 alpha in breast epithelial cells. PLoS ONE 6(3):1–10. https://doi.org/10.1371/journal.pone.0017563
Vermeulen MA, Slaets L, Cardoso F et al (2017) Pathological characterisation of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Eur J Cancer 82(March):219–227. https://doi.org/10.1016/j.ejca.2017.01.034
Matsumura T, Endo T, Isotani A et al (2019) An azoospermic factor gene, Ddx3y and its paralog, Ddx3x are dispensable in germ cells for male fertility. J Reprod Dev 65(2):121–128. https://doi.org/10.1262/jrd.2018-145
Chan CH, Chen CM, Lee YHW et al (2019) DNA damage, liver injury, and tumorigenesis: consequences of DDX3X loss. Mol Cancer Res 17(2):555–566. https://doi.org/10.1158/1541-7786.MCR-18-0551
Lee CH, Lin SH, Yang SF et al (2014) Low/negative expression of DDX3 might predict poor prognosis in non-smoker patients with oral cancer. Oral Dis 20(1):76–83. https://doi.org/10.1111/odi.12076
Hwang K, Kim YA, Kim J et al (2020) Influence of androgen receptor on the prognosis of breast cancer. J Clin Med 9(4):1083. https://doi.org/10.3390/jcm9041083
Vellky JE, McSweeney ST, Ricke EA et al (2020) RNA-binding protein DDX3 mediates posttranscriptional regulation of androgen receptor: a mechanism of castration resistance. Proc Natl AcadSci U S A 117(45):28092–28101. https://doi.org/10.1073/pnas.2008479117
Lash TL, Fox MP, Westrup JL et al (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220. https://doi.org/10.1007/s10549-006-9193-0
Acknowledgements
Natalie D. ter Hoeve did all the immunohistochemistry.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Carmen C. van der Pol, Cathy B. Moelans, Quirine F. Manson and Marilot C.T. Batenburg. The first draft of the manuscript was written by Carmen C. van der Pol and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Use of archival tissue and data was done according to the General Data Protection Regulation and Dutch law.
Conflict of interest
V. Raman and P.J. van Diest hold a patent on DDX3. No potential conflicts of interest were disclosed by the other authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Pol, C.C., Moelans, C.B., Manson, Q.F. et al. Cytoplasmic DDX3 as prognosticator in male breast cancer. Virchows Arch 479, 647–655 (2021). https://doi.org/10.1007/s00428-021-03107-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03107-4