Abstract
Standardized structured reporting (SSR) enables high-quality pathology reporting, but implementing SSR is slow. The objective of this study is to identify both barriers and facilitators that pathologists encounter in SSR, in order to develop tailored implementation tools to increase SSR usage. We used a mixed method design: a focus group interview helped to identify barriers and facilitators in SSR. The findings were classified into the following domains: innovation, individual professional, social setting, organization, and economic and political context. We used a web-based survey among Dutch pathologists to quantify the findings. Ten pathologists participated in the focus group interview, and 97 pathologists completed the survey. The results of both showed that pathologists perceive barriers related to SSR itself. Particularly its incompatibility caused lack of nuance (73%, n = 97) in the standardized structured pathology report. Regarding the individual professional, knowledge about available SSR-templates was lacking (28%, n = 97), and only 44% (n = 94) of the respondents agreed that using SSR facilitates the most accurate diagnosis. Related to social setting, support from the multidisciplinary team members was lacking (45%, n = 94). At organization level, SSR leads to extra work (52%, n = 94) because of its incompatibility with other information systems (38%, n = 93). Main facilitators of SSR were incorporation of speech recognition (54%, n = 94) and improvement in communication during multidisciplinary team meetings (69%, n = 94). Both barriers and facilitators existed in various domains. These factors can be used to develop implementation tools to encourage SSR usage.
Similar content being viewed by others
Introduction
Discussion of each patient in multidisciplinary team (MDT) meetings is fundamental to optimal treatment decisions [1]. The complexity of modern multidisciplinary oncological diagnostics and treatment warrants timely, complete, adequate, and accurate exchange of information based on pathology reports. Optimization of pathology reports may save time and money [2].
The use of standardized structured reporting (SSR) in pathology instead of the traditional narrative, free-text reporting (NR) improves exchange of diagnostic and treatment information [3]. As a consequence of improved communication, MDT-members can take more appropriate treatment decisions, which leads to improved patient outcomes [4,5,6,7,8]. Multiple national and international guidelines, including those of the College of American Pathologists (CAP) [9], the Royal College of Pathologists (RCP) [10], the Dutch guidelines [11], and International Collaboration on Cancer Reporting (ICCR) [12], therefore recommend SSR. SSR includes an electronic reporting template with standardized reporting language, multiple-choice answering of parameters, and automated conclusion generation, which leads to well-structured overviews of the essential parameters of specific cases [13]. SSR is also important to secondary users including tumor registry organizations, health planners, epidemiologists, and others involved in healthcare quality-improvement activities and research [13,14,15,16,17,18].
The use of SSR in pathology varies widely across laboratories and tumor types [19,20,21,22,23]. In the Netherlands, we introduced SSR in 2009. After 9 years of implementation, SSR is now used in 86% of the colorectal cancer resections and 81% of the breast cancer resections. Over the years, SSR was introduced for another 20 cancer types, resulting in 27 different SSR-templates in total. The actual use of the SSR-templates varied from 8 to 86% (median 54%) in 2017 [24]. The wide range of actual SSR use in the Netherlands is comparable to other countries [19,20,21,22,23]. Therefore, despite some success with passive implementation, there is considerable room for improvement to the extent and speed of implementing SSR in pathology.
The adoption of innovations such as SSR is laborious, particularly when innovation requires people to change their daily habits [25]. Offering implementation tools facilitates adoption of innovations among medical professionals. Implementation tools are most effective when they take into account factors that impede or facilitate use of the innovation [26,27,28]. Therefore, the aim of this study was to explore barriers and facilitators in SSR-implementation among pathologists in a nationwide setting.
Methods
Study design
We used a mixed method design. We used a qualitative focus group interview to identify barriers and facilitators that Dutch pathologists encountered in SSR. We used a web-based survey to quantify the findings: to identify possible determinants and to assess the importance of the barriers and facilitators.
Setting
PALGA, the nationwide network and registry of histo- and cytopathology in the Netherlands, was established in 1971 and achieved nationwide coverage in 1991, which means that all 46 Dutch pathology laboratories are connected to the PALGA network. The PALGA database contains the reports of all pathology tests in the Netherlands. This database is used for supporting patient care, for evaluating and monitoring the population screening programs, and for scientific research purposes [29]. More detailed information about PALGA can be found in additional file 1.
PALGA introduced SSR by providing their nationally available SSR-templates [30]. The Dutch SSR-templates are electronic reporting templates based on national evidence-based guidelines and WHO guidelines [31], with standardized reporting language, multiple-choice answering of parameters, and automated conclusion generation, generating a standardized structured pathology report. At the time of data collection, 21 nationwide SSR-templates were available. The use of the colon biopsy SSR template is mandatory for registering data for the national screening program for bowel cancer [24]. The PALGA SSR-templates are considered level 6, the highest level of the Spectrum of Cancer Pathology Reporting [19]. In additional file 2, the PALGA SSR-template of breast cancer biopsy is shown. To accurately refer to this level 6 type of reporting, we chose to use the term “standardized structured reporting” (SSR).
Other important pathology associations are the Dutch Society of Pathology (NVVP) and the Dutch Society of Pathology Residents (LPAV). Both pathologists and residents are members of the NVVP, whereas the LPAV only allows residents. At the time of data collection, 395 pathologists [32] and 105 residents were active in the Netherlands [33].
Participants
Focus group interview
One of our researchers (CS) purposively e-mailed 30 pathologists. We aimed to include pathologists from university and non-university hospitals with different lengths of experience, as well as the range of opinion leaders, middle majority, and stragglers regarding SSR. The participating pathologists filled in a short questionnaire consisting of questions about the pathologists’ characteristics, clinical setting, and SSR usage. They also signed informed consent forms for recording the focus group interview and for anonymously analyzing the data obtained.
Survey
We invited and reminded pathologists to complete the survey via the PALGA network. The PALGA liaisons distributed the survey to their pathologist colleagues. Further, a call to complete the survey was posted on the PALGA webpage (www.palga.nl/nieuws), on the PALGA LinkedIn page, and in the newsletters from the Dutch Society of Pathology Residents and the Dutch Society of Pathology for 4 consecutive weeks. The introduction of the questionnaire informed the respondents about the research question and the anonymous data analysis. Completing the questionnaire took 10 to 15 min.
Instrument development and data collection
Focus group interview
We used the domains of the theoretical models of Flottorp et al. [28] and Grol and Wensing [27] to develop an interview guide, with which we structured the focus group interview. The focus group interview took place at the annual PALGA day. An independent chairman, former head of the Radboudumc pathology department, supervised the focus group. The researcher (CS) started the focus group interview with an explanation of the research question. Then participants introduced themselves by explaining their sub-specialism within pathology, if applicable, and their experience with SSR. Next, the chairman asked about existing barriers and facilitators in the various domains. At the end, participants had time for final remarks. The interview was audio-taped and transcribed verbatim for content analysis with Atlas.ti (version 7.5.15, Atlas.ti Scientific Software Development; Berlin, Germany).
Survey
The survey was developed on the basis of the barriers and facilitators in SSR-implementation identified in the focus group interview, and some factors were added from literature as well [27, 28]. We used LimeSurvey (version 2.06+) to develop a web-based survey. The online survey did not accept unanswered questions, but the respondent could end the survey at any given time. The first part of the survey contained questions about the pathologists’ characteristics and their clinical settings. The second part of the survey consisted of seven theses and one open-ended question about SSR usage, as well as 61 theses to quantify the barriers and facilitators in SSR-implementation (both the use of SSR-templates and standardized structured pathology reports). These theses were classified in five main domains. To prevent repeated answers for all items, some theses were formulated in reverse. The theses were scored on a five-point Likert scale (strongly disagree (0) to strongly agree (4)), and when relevant, “Some do, others do not” (2) was included. A high mean score implies a facilitator, whereas a low score implies a barrier. At the end of the survey, participants could state their preferences, name additional barriers and facilitators, and suggest improvements for SSR-implementation.
Outcome measures
The primary outcome measure was the set of identified barriers and facilitators for implementing SSR. The secondary outcome measures were determinants associated with barriers and facilitators.
Data analyses
Focus group interview
Two researchers (CS and LO) independently extracted barriers and facilitators in SSR-implementation from the transcribed interview. The factors identified were allocated to the five domains. The two researchers discussed any discrepancies until they achieved consensus.
Survey
We used IBM SPSS Statistics 24 to analyze the survey results. We included questionnaires with at least 50% of the answers completed [34]. We used descriptive statistics to analyze participant characteristics and to quantify agreement with the theses in the survey. We categorized the agreement scores as “strongly agree,” “agree,” and “strongly disagree.” The category “strongly disagree” includes the answer option “Some do, some don’t.” Because this was a self-developed survey, we used psychometric properties (Cronbach’s α) and component analyses to confirm the reliability of the questionnaire. We turned negative items around to calculate Cronbach’s α and mean scores. We found high Cronbach’s α’s in all domains or sub-domains; credibility (.85), feasibility (.89), compatibility (.76), knowledge and skills (.95), attitude (.75), social setting (.80), and organizational factors (.74). We deemed the questionnaire reliable by these results. We used conventional content analysis to analyze the qualitative comments in the survey [35].
In analyzing possible determinants at domain level, we used Cronbach’s α to check the consistency of the domain and calculate the mean score for each domain. Further, we employed multivariable linear regression analyses to investigate the existence of possible determinants of barriers and facilitators identified [36]. Based on literature and differences in agreement with the barriers and facilitators in SSR-implementation noted in the focus group interview, we investigated various determinants: pathologist and hospital characteristics, such as age, gender, career stage, number of colleagues within the pathology department; and employment at a university or non-university hospital. We first examined the associations between one determinant and all dependent variables (i.e., domains of influencing factors) with univariate linear regression analyses. We included only those determinants associated with the dependent variables (two-sided p ≤ 0.20) in the multivariable linear regression analyses.
Results
Study population
Ten pathologists participated in the focus group interview—five men and five women—with an average of 14.9 years of experience (3–30 years). Three pathologists worked in a university hospital and seven in a non-university hospital.
The survey yielded 119 responses, of which 97 had at least the minimum number of answers required (82%). Table 1 outlines the participant characteristics. The mean number of years of experience as a pathologist was 11.0, ranging from 0.2 to 30.0 years.
Use of SSR
Of the survey respondents, 82% (n = 97) frequently used SSR. On average, 61% of the SSR-templates were used. The main reasons for not using SSR-templates were non-availability for specific tumors, the teaching setting of the laboratory, lack of awareness of available templates, lack of nuances in SSR, and the perceived extra time necessary for SSR. Most respondents (68%; n = 93) preferred SSR, 18% (n = 93) preferred NR, 4% (n = 93) preferred a local template, and 10% (n = 93) had no opinion. Furthermore, 28% (n = 97) lacked knowledge about the available SSR-templates. Most pathologists received information about updates of SSR-templates via their PALGA liaison (56%; n = 97).
Barriers and facilitators
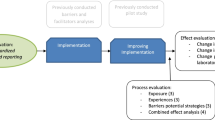
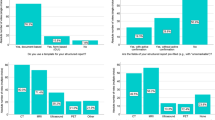
Thirty-two barriers and 29 facilitators in SSR-implementation were identified in the focus group interview. Thirteen factors were considered to be both barriers and facilitators. Figure 1 illustrates the barriers and facilitators in SSR-implementation noted in the focus group by domain. All influencing factors were used in the web-based survey. Figures 2 and 3 show the survey answers. The most remarkable barriers and facilitators in SSR-implementation are discussed by domain.
Innovation: SSR
Pathologists mentioned influencing factors related to the credibility, feasibility, and compatibility of SSR (Fig. 2). First, SSR-templates did not facilitate a speedy draft of the pathology report (36%; n = 94). However, 82% (n = 97) of the respondents acknowledged the added value of SSR, since it facilitates a uniform (97%; n = 94) and complete (93%; n = 94) pathology report.
Second, feasibility of SSR depended on the type of specimen. SSR-templates are inadequate for the reporting of multiple specimens per patient (69%; n = 97), for reporting revisions or consultations (70%; n = 97), and for reporting biopsies (29%; n = 97). However, SSR-templates are feasible for 90% of the routine diagnostics (78%; n = 97), including large resections (84%; n = 97), and, in contrast to the focus group findings, even after neoadjuvant therapy (80%; n = 97). SSR could be used by all pathologists, but are more attractive to pathologists without a sub-specialization (90%; n = 97). Furthermore, SSR-templates are supportive tools, either a checklist (96%; n = 97) or a calculator for cut-off points (91%; n = 97).
Third, SSR provided the respondents with difficulties in identifying relevant slides in colleagues’ standardized structured pathology reports (95%; n = 97), lacked nuances in these pathology reports (73%; n = 97), and showed poor report readability (53%; n = 95). In addition, the faltering of follow-up questions when clicking was undesirable (64%; n = 94). However, 83% (n = 94) stated that SSR were compatible with current practices. Specifically, SSR should not include only mandatory items (84%; n = 97) because it was helpful to be able to add extra information to non-mandatory items (85%; n = 94). The pathologists also agreed with these mandatory items (78%; n = 97). If speech recognition was added to the SSR, 54% (n = 94) would use it more often.
Individual professional
Figure 3 reveals the items in the domains of the individual professional, social setting, organization, and economic and political context. The respondents were familiar with SSR (94%; n = 94) and had sufficient knowledge (94%; n = 94) and skills (96%; n = 94) for using SSR. With regard to attitude, only 44% (n = 94) agreed that the use of SSR facilitated the most accurate diagnosis, and 46% (n = 97) agreed that black-and-white conclusions in generalized terms are undesirable. However, all respondents were in favor of guidelines, and 97% (n = 94) were also in favor of SSR and motivated to use them (86%; n = 94). Uniformity of the pathology report is important (86%; n = 94).
Social setting
Forty-five percent (n = 94) of the survey respondents lacked support from MDT-members to use SSR. However, SSR facilitated communication within the multidisciplinary team (69%; n = 94). The focus group participants mentioned that clinicians receiving the standardized structured pathology report had to become familiar with this new way of reporting and did not universally agree with the SSR format. The survey respondents stated that superiors (84%; n = 94) and colleagues in the pathology department (78%; n = 94) encouraged SSR and considered it useful in MDT-meetings (78%; n = 94).
Organizational factors
According to 33% of the respondents (n = 93), SSR was considered inappropriate for the education of residents. Both the focus group interview and survey respondents (52%; n = 94) said that using SSR leads to more work because data must be entered into multiple systems. In addition, SSR was incompatible with other departmental software systems (38%; n = 93). However, the quality systems of the laboratories did not affect the use of SSR (89%; n = 93). Sufficient financial resources were available to the laboratories to use SSR (97%; n = 93), and using SSR also had high priority in the respondents’ pathology departments (78%; n = 94). Furthermore, PALGA delivered appropriate information about changes after a SSR update and development of new SSR (84%; n = 93).
Economic and political context
The participants did not name any barriers in the economic and political context during the focus group interview. The respondents (87%; n = 94) reported that a mandatory SSR-template, such as for the national screening program for bowel cancer, is a facilitator.
Improvements for SSR
Proposed improvements for SSR entailed improving the readability, nuance, and adaptability of the generated report; improving compatibility with other hospital IT systems; reducing or increasing the mandatory minimal dataset; and incorporating speech recognition software for both the main content and additional text fields.
Determinants of the barriers and facilitators in SSR
The mean scores for all domains and sub-domains were above 2.1 (Likert scale 0–4) (Table 2). Univariate determinant analyses resulted in multiple determinants at professional and hospital level for (1) credibility, (2) knowledge and skills, and (3) social setting. These were gender and years of experience (p = .191 and p = .191), career stage and type of hospital (p = .034 and p = .090), and being a specialist or not and type of hospital (p = .138 and p = .182), respectively. Additionally, determinants for compatibility and attitude were found. These were being a specialist or not (p = .056) and gender (p = .151), respectively. After multivariable analyses (Table 2), career stage remained a possible determinant for knowledge and skills of SSR (mean scores: residents, 2.98; pathologists, 3.38; p = .034); pathologists’ career stage had an influence on the knowledge and skills of pathologists to work with SSR.
Discussion
This study has uncovered several important factors in the implementation of SSR in all domains of the theoretical models of Flottorp et al. [28] and Grol and Wensing [27]. The main barriers were usually related to infeasibility and incompatibility of SSR with pathologists’ current practices. Other important barriers were lack of knowledge about available SSR-templates, disbelief about the capability to deliver the most accurate diagnosis, disencouragement of SSR usage by MDT-members, and repetitive entries for multiple systems. The main facilitators related to multiple domains and sub-domains as well. The most important facilitators were incorporation of speech recognition in SSR, SSR uniformity of pathology reports, having superiors encouraging SSR, improved communication during MDT-meetings, and the mandatory SSR use for registering colon biopsy in the national screening program for bowel cancer. The only determinant of SSR-implementation appeared to be career stage. This is a minor contrast to earlier studies, that identified age rather than career stage as a determinant of implementation [14, 15, 20, 37]. The influencing factors of the innovation (SSR) and the individual professional factors are most relevant in settings with a high level SSR (level 6 of the Spectrum of Cancer Pathology Reporting [19] or higher). Moreover, the influencing social setting factors, both in the laboratory and MDT-setting, are relevant in countries that have multidisciplinary health care organization. Full use of the influencing organizational and political and economic context factors can be made in the setting of national or regional organizations that coordinate SSR.
Pathologists’ reluctance to implement SSR is visible in multiple studies [22, 23, 37,38,39,40]. Our study confirms previous findings that incompatibility with pathologists’ current practices is an important barrier [39]. Interestingly, most barriers related to the use of the standardized structured pathology report rather than the SSR-template. Before improving the readability, nuance, and adaptability of the standardized structured pathology report as the pathologists in this study and Ellis and Srigley [13] proposed, other MDT-members’ perceptions of compatibility of the standardized structured pathology report should been taken into account. The study by Lankshear et al. [38] shows a correlation between clinicians’ satisfaction and the information level along with easy information retrieval needed for patient management. Therefore, future research should aim at the clinicians’ perceptions of the Dutch SSR system to determine whether MDT-members perceive barriers in the standardized structured pathology report. If MDT-members do not face barriers relating to incompatibility of the standardized structured pathology report, they may play an important role in encouraging SSR use among pathologists [28].
An important barrier of SSR is an increased workload due to multiple incompatible systems, which require additional input of data [22, 23, 38]. Hassell and colleagues [23] propose adapting the pathologist workflow and reporting format as a solution. Additional research, such as an explorative focus group interview with the PALGA liaisons, is needed to clarify the barriers to multiple system use.
Multiple studies have published findings regarding the improved overall completeness of a pathology report when SSR (defined by Ellis and Srigley [13] as level 3 or higher) instead of NR, is used to report pathology evaluation [14, 15, 17, 19, 20, 22, 41,42,43,44,45,46]. In this study, we assume that the use of SSR facilitates a complete and uniform report. Future research should explore whether the end-users of the pathology SSR, indeed, classify the standardized structured pathology reports as complete and adequate for treatment decision making.
Incorporation of the findings from the focus group interview and survey in the implementation strategy of SSR should be used to develop a tailored implementation strategy. First, communication about availability of SSR for specific tumors should be improved, by providing clear guidelines to the PALGA liaison’s in the laboratories.
Second, to improve the feasibility and compatibility of SSR for pathologists, there should be a focus on training; for example, by e-learning or instruction videos [28]. The positive effects of such implementation training sessions about SSR usage has been observed in multiple studies [17, 22, 47, 48]. Most importantly, the use of SSR-templates in cases of multiple resection specimens should be discussed. Because residents have less knowledge and skills to work with SSR, the training program could be adapted to career stage.
Third, to overcome the negative attitudes of pathologists not using SSR, Srigley et al. [19] successfully used multiple funding sources as incentives for increasing the use of SSR in Ontario. Hassell et al. [23] suggest financial benefits, for example, grant programs, to encourage SSR use among pathologists. This study shows that mandatory registration was an important facilitator. Therefore, the incentive of additional funding of SSR could be discussed among secondary users of SSR who benefit from SSR.
Fourth, another important barrier to address is the use of multiple software programs in laboratories, which interrupts the workflow of pathologists and requires more time due to repetitive data entry. As Hassell and colleagues propose, the different software programs used to complete the standardized structured pathology report could be more integrated to facilitate a more efficient workflow and registry process. Speech recognition could be incorporated in SSR-templates for both the main content and additional text fields. Further, clinical data and macroscopy findings may be transferred automatically in the SSR-template. In this way, data is registered only once at the source and can be used multiple times.
Strengths and limitations
The use of both qualitative and quantitative methods is a strength in our study. We selected the participants of the focus group with care to minimize the selection bias. This resulted in a diverse group including opinion leaders, middle majority, and stragglers. Our intention was to gain a view as broad as possible of any influencing factors. Additionally, we conducted a web-based survey among all pathologists in the Netherlands to quantify the barriers and facilitators in SSR named in the focus group. This enabled us to determine the most important barriers and facilitators.
There are some limitations as well. Due to the open invitation to the web-based questionnaire, we could not calculate the response rate. With 97 questionnaires, we may have some selection bias in our web-based survey. However, the main characteristics (gender, age, personnel type, and type of hospital) of the respondents represent the Dutch population of pathologists and pathology residents. In addition, the results showed that the respondent preferences were diverse, and even when pathologists preferred SSR, they still mentioned barriers. Moreover, only pathologists were included in this study. The pathology report readers, who are clinicians such as oncologists, surgeons, and radiologists, should be included to obtain a complete overview of the possible influencing factors. These clinicians are part of the MDT that uses the standardized structured pathology reports to take treatment decisions.
In conclusion, this study identified barriers to and facilitators for implementing SSR among pathologists in the Netherlands. Implementation tools based on these factors could increase the extent and speed of SSR-implementation. We would recommend investigating barriers to and facilitators for SSR-implementation among clinicians receiving pathology reports such as oncologists, surgeons, and radiologists to further increase the use of SSR.
References
Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, Crowe J, Costello AJ (2016) The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev 42:56–72. https://doi.org/10.1016/j.ctrv.2015.11.007
Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, Ramirez AJ (2010) Multidisciplinary team working in cancer: what is the evidence. BMJ 340:c591
Sluijter CE, van Lonkhuijzen LRCW, van Slooten HJ, Nagtegaal ID, Overbeek LI (2016) The effects of implementing synoptic pathology reporting in cancer diagnosis: a systematic review. Virchows Arch 468(6):639–649. https://doi.org/10.1007/s00428-016-1935-8
MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, Porteous C (2009) Improving patient survival with the colorectal cancer multi-disciplinary team. Color Dis 11(3):291–295. https://doi.org/10.1111/j.1463-1318.2008.01580.x
Wille-Jørgensen P, Sparre P, Glenthøj A, Holck S, Nørgaard Petersen L, Harling H, Stub Højen H, Bülow S (2013) Result of the implementation of multidisciplinary teams in rectal cancer. Color Dis 15(4):410–413. https://doi.org/10.1111/codi.12013
Palmer G, Martling A, Cedermark B, Holm T (2011) Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Color Dis 13(12):1361–1369. https://doi.org/10.1111/j.1463-1318.2010.02460.x
Sluijter CE, van Workum F, Wiggers T, van de Water C, Visser O, van Slooten HJ, Overbeek LIH, Nagtegaal ID (2019) Improvement of care in patients with colorectal cancer: influence of the introduction of standardized structured reporting for pathology. JCO CCI 3:1–12. https://doi.org/10.1200/cci.18.00104
Bydder S, Nowak A, Marion K, Philiips M, Atun R (2009) The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J 39(12):838–841. https://doi.org/10.1111/j.1445-5994.2009.02019.x
Pathologists, C.o.A. (2016) Cancer protocol templates. www.cap.org/. Accessed 14 November 2016
RCP (2014) Standards and datasets for reporting cancers, in Dataset for colorecal cancer histopathology reports. file:///G:/Downloads/Dataset%20for%20colorectal%20cancer%20histopathology%20reports%20(3rd%20edition).pdf. Accessed 14 November 2016
Oncoline (2018) Dutch guidelines for breast cancer. http://www.oncoline.nl/borstkanker. Accessed 4 June 2018
Reporting, I.C.o.C (2017) Datasets for a consistent, evidence bases approach for reporting of cancer. http://www.iccr-cancer.org/datasets. Accessed 18 April 2017
Ellis DW, Srigley J (2016) Does standardised structured reporting contribute to quality in diagnostic pathology? The importance of evidence-based datasets. Virchows Arch 468(1):51–59. https://doi.org/10.1007/s00428-015-1834-4
Aumann K, Amann D, Gumpp V, Hauschke D, Kayser G, May AM, Wetterauer U, Werner M (2012) Template-based synoptic reports improve the quality of pathology reports of prostatectomy specimens. Histopathology 60(4):634–644. https://doi.org/10.1016/j.lungcan.2013.05.017
Aumann K, Kayser G, Amann D, Bronsert P, Hauschke D, Palade E, Passlick B, Werner M (2013) The format type has impact on the quality of pathology reports of oncological lung resection specimens. Lung Cancer 81(3):382–387. https://doi.org/10.1016/j.lungcan.2013.05.017
Aumann K, Niermann K, Asberger J, Wellner U, Bronsert P, Erbes T, Hauschke D, Stickeler E, Gitsch G, Kayser G, Werner M (2016) Structured reporting ensures complete content and quick detection of essential data in pathology reports of oncological breast resection specimens. Breast Cancer Res Treat 156(3):495–500. https://doi.org/10.1007/s10549-016-3769-0
Hammond EH, Flinner RL (1997) Clinically relevant breast cancer reporting: using process measures to improve anatomic pathology reporting. Arch Pathol Lab Med 121(11):1171–1175
Srigley JR, Lankshear S, Brierly J, McGowan T, Divaris D, Yurcan M, Rossi R, Yardley T, King MJ, Ross J, Irish J, McLeod R, Sawka C (2013) Closing the quality loop: facilitating improvement in oncology practice through timely access to clinical performance indicators. J Oncol Pract 9(5):e255–e261 http://ascopubs.org/doi/10.1200/JOP.2012.000818
Srigley JR, McGowan T, Maclean A, Raby M, Ross J, Kramer S, Sawka C (2009) Standardized synoptic cancer pathology reporting: a population-based approach. J Surg Oncol 99(8):517–524. https://doi.org/10.1002/jso.21282
Casati B, Bjugn R (2012) Structured electronic template for histopathology reporting on colorectal carcinoma resections: five-year follow-up shows sustainable long-term quality improvement. Arch Pathol Lab Med 136(6):652–656. https://doi.org/10.5858/arpa.2011-0370-OA
Haugland HK, Casati B, Dørum Mtech LM, Bjugn R (2011) Template reporting matters--a nationwide study on histopathology reporting on colorectal carcinoma resections. Hum Pathol 42(1):36–40. https://doi.org/10.1016/j.humpath.2010.06.009
Branston LK, Greening S, Newcombe RG, Daoud R, Abraham JM, Wood F, Dallimore NS, Steward J, Rogers C, Williams GT (2002) The implementation of guidelines and computerised forms improves the completeness of cancer pathology reporting. The CROPS project a randomised controlled trial in pathology. Eur J Cancer 38(6):764–772. https://doi.org/10.1016/S0959-8049(01)00258-1
Hassell LA, Parwani AV, Weiss L, Jones MA, Ye J (2010) Challenges and opportunities in the adoption of College of American Pathologists checklists in electronic format: perspectives and experience of Reporting Pathology Protocols Project (RPP2) participant laboratories. Arch Pathol Lab Med 134(8):1152–1159
PALGA (2018) Stichting PALGA. https://www.palga.nl/. Accessed 15 June 2018
Grol R, Grimshaw J (1999) Evidence-based implementation of evidence-based medicine. Jt Comm J Qual Improv 25(10):503–513
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR (1999) Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 282(15):1458–1465
Grol R, Wensing M (2004) What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust 180(6 Suppl):S57–S60. https://doi.org/10.5694/j.1326-5377.2004.tb05948.x
Flottorp SA, Oxman AD, Krause J, Musila NR, Wensing M, Godycki-Cwirko M, Baker R, Eccles MP (2013) A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 8:35. https://doi.org/10.1186/1748-5908-8-35
Casparie M, Tiebosch AT, Burger G, Blauwgeers H, Van de Pol A, van Krieken AH, Meijer GA (2007) Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29(1):19–24
Williams CL, Bjugn R, Hassell LA (2015) Current status of discrete data capture in synoptic surgical pathology and cancer reporting. Pathol Lab Med Int 7:11–22
Netherlands, C.C.C.t (2016) Oncoline; Cancer Clinical Practice Guideline. http://www.oncoline.nl/index.php?language=en Accessed 2 November 2016
CBS StatLine. (2018) Medisch geschoolden; arbeidspositie, positie in de werkkring, naar beroep. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81551NED/table?ts=1558090615149. Accessed 17 May 2019
Dutch society of pathologists. (2018) Manpower analyse 2017v2. https://pathology.nl/wp-content/uploads/2018/03/Manpower-analyse-2017v2.pdf. Accessed 17 May 2019
Zorgverzekeringen, C.v. (2013) Werkinstructie Het opschonen van data bij schriftelijke en of online dataverzameling. https://www.zorginzicht.nl/kennisbank/Documents/CQI-handboek-controleren/Werkinstructie-06.01-Het-opschonen-van-data-bij-schriftelijke-dataverzameling.pdf. Accessed 11 April 2018
Hsieh HF, Shannon SE (2005) Three approaches to qualitative content analysis. Qual Health Res 15(9):1277–1288. https://doi.org/10.1177/1049732305276687
Geurts-Laurant M, Hermens RPMG, Braspenning JCC, Sibbald B, Grol R (2004) Impact of nurse practitioners on workload of general practitioners: randomised controlled trial. BMJ 328(7445):927. https://doi.org/10.1136/bmj.38041.493519.EE
Hassell LA, Aldinger W, Moody C, Winters S, Gerlach K, Schwenn M, Perriello D (2009) Electronic capture and communication of synoptic cancer data elements from pathology reports: results of the Reporting Pathology Protocols 2 (RPP2) project. J Registry Manag 36(4):117–124 quiz 163–165
Lankshear S, Srigley J, McGowan T, Yurcan M, Sawka C (2013) Standardized synoptic cancer pathology reports - so what and who cares? A population-based satisfaction survey of 970 pathologists, surgeons, and oncologists. Arch Pathol Lab Med 137(11):1599–1602. https://doi.org/10.5858/arpa.2012-0656-OA
Urquhart R, Porter GA, Sargeant J, Jackson L, Grunfeld E (2014) Multi-level factors influence the implementation and use of complex innovations in cancer care: a multiple case study of synoptic reporting. Implement Sci 9:121. https://doi.org/10.1186/s13012-014-0121-0
Services UDohah (2009) Electronic reporting in pathology: requirements and limitations. A Paradigm for National Electronic Health Records Implementation, Washington, DC
Siriwardana PN, Pathmeswaran A, Hewavisenthi J, Deen KI (2009) Histopathology reporting in colorectal cancer: a proforma improves quality. Color Dis 11(8):849–853
Appleton MA, Douglas-Jones AG, Morgan JM (1998) Evidence of effectiveness of clinical audit in improving histopathology reporting standards of mastectomy specimens. J Clin Pathol 51(1):30–33
Idowu MO, Bekeris LG, Raab S, Ruby SG, Nakhleh RE (2010) Adequacy of surgical pathology reporting of cancer: a College of American Pathologists Q-Probes study of 86 institutions. Arch Pathol Lab Med 134(7):969–974
Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O’Connell D (2012) Pathways to the diagnosis of thyroid cancer in New South Wales: a population-based cross-sectional study. Cancer Causes Control 23(1):35–44. https://doi.org/10.1007/s10552-011-9852-2
Mathers ME, Shrimankar J, Scott DJ, Charlton FG, Griffith CDM, Angus B (2001) The use of a standard proforma in breast cancer reporting. J Clin Pathol 54(10):809–811
Renshaw SA, Mena-Allauca M, Touriz M, Renshaw A, Gould EW (2014) The impact of template format on the completeness of surgical pathology reports. Arch Pathol Lab Med 138(1):121–124
Chan NG, Duggal A, Weir MM, Driman DK (2008) Pathological reporting of colorectal cancer specimens: a retrospective survey in an academic Canadian pathology department. Can J Surg 51(4):284–288
Ihnát P, Delongová P, Horáček J, Ihnát Rudinská L, Vávra P, Zonča P (2015) The impact of standard protocol implementation on the quality of colorectal cancer pathology reporting. World J Surg 39(1):259–265. https://doi.org/10.1007/s00268-014-2796-4
Acknowledgments
We thank all pathologists who participated in the focus group interview and survey. Especially, we thank Prof. D. J. Ruiter, MD, PhD, for being the chairman of the focus group interview.
Contribution statement
Author C, D, and E conceived and designed the study, and wrote, edited, and reviewed the manuscript. Author B and C collected and analyzed the data and wrote, edited, and reviewed the manuscript. Author A analyzed the data, and wrote, edited, and reviewed the manuscript. All authors gave final approval for publication. Author E takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Funding
This study was funded by the Dutch Cancer Society/Alpe d’huzes (grant number KUN 2013-6354).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study is carried out in the Netherlands in accordance with the applicable rules concerning informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ms. Swillens and Ms. Sluijter were lead contributors for this article
This article is part of the Topical Collection on Quality in Pathology
Electronic supplementary material
Additional file 1
General information about the PALGA foundation (PDF 7 kb)
Additional file 2
Screenshots of the Dutch PALGA SSR-template of breast cancer biopsy (translated from Dutch to English) (PDF 448 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Swillens, J.E.M., Sluijter, C.E., Overbeek, L.I.H. et al. Identification of barriers and facilitators in nationwide implementation of standardized structured reporting in pathology: a mixed method study. Virchows Arch 475, 551–561 (2019). https://doi.org/10.1007/s00428-019-02609-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02609-6