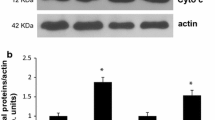
Abstract
Autophagy and mitophagy are important for training-inducible muscle adaptations, yet it remains unclear how these systems are regulated throughout the adaptation process. Here, we studied autophagic and mitophagic flux in the skeletal muscles of Sprague–Dawley rats (300–500 g) exposed to chronic contractile activity (CCA; 3 h/day, 9 V, 10 Hz continuous, 0.1 ms pulse duration) for 1, 2, 5, and 7 days (N = 6–8/group). In order to determine the flux rates, colchicine (COL; 0.4 mg/ml/kg) was injected 48 h before tissue collection, and we evaluated differences of autophagosomal protein abundances (LC3-II and p62) between colchicine- and saline-injected animals. We confirmed that CCA resulted in mitochondrial adaptations, including improved state 3 respiration as early as day 1 in permeabilized muscle fibers, as well significant increases in mitochondrial respiratory capacity and marker proteins in IMF mitochondria by day 7. Mitophagic and autophagic flux (LC3-II and p62) were significantly decreased in skeletal muscle following 7 days of CCA. Notably, the mitophagic system seemed to be downregulated prior (day 3–5) to changes in autophagic flux (day 7), suggesting enhanced sensitivity of mitophagy compared to autophagy with chronic muscle contraction. Although we detected no significant change in the nuclear translocation of TFEB, a regulator of lysosomal biogenesis, CCA increased total TFEB protein, as well as LAMP1, in skeletal muscle. Thus, chronic muscle activity reduces mitophagy in parallel with improved mitochondrial function, and this is supported by enhanced lysosomal degradation capacity.





Similar content being viewed by others
References
Adhihetty PJ, Ljubicic V, Hood DA (2007) Effect of chronic contractile activity on SS and IMF mitochondrial apoptotic susceptibility in skeletal muscle. Am J Physiol Endocrinol Metab 292:E748–E755. https://doi.org/10.1152/ajpendo.00311.2006
Ai H, Ralston E, Lauritzen HP, Galbo H, Ploug T (2003) Disruption of microtubules in rat skeletal muscle does not inhibit insulin- or contraction-stimulated glucose transport. Am J Physiol Endocrinol Metab 285:E836–E844. https://doi.org/10.1152/ajpendo.00238.2002
Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC (2016) Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8:127–146. https://doi.org/10.18632/aging.100879
Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H (2018) Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Phys Rep 6:e13651. https://doi.org/10.14814/phy2.13651
Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA (2018) Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol 596:3567–3584. https://doi.org/10.1113/JP275998
Chen CCW, Erlich AT, Hood DA (2018) Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet Muscle 8:10. https://doi.org/10.1186/s13395-018-0157-y
Erlich AT, Brownlee DM, Beyfuss K, Hood DA (2018) Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1alpha-dependent manner. Am J Phys Cell Physiol 314:C62–C72. https://doi.org/10.1152/ajpcell.00162.2017
Fletcher LM, Welsh GI, Oatey PB, Tavare JM (2000) Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake. Biochem J 352(Pt 2):267–276
Halling JF, Ringholm S, Nielsen MM, Overby P, Pilegaard H (2016) PGC-1alpha promotes exercise-induced autophagy in mouse skeletal muscle. Phys Rep 4:e12698. https://doi.org/10.14814/phy2.12698
Hood DA (2001) Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol (1985) 90:1137–1157
Hood DA, Tryon LD, Carter HN, Kim Y, Chen CC (2016) Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J 473:2295–2314. https://doi.org/10.1042/BCJ20160009
Ju JS, Jeon SI, Park JY, Lee JY, Lee SC, Cho KJ, Jeong JM (2016) Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci 66:417–430. https://doi.org/10.1007/s12576-016-0440-9
Ju JS, Varadhachary AS, Miller SE, Weihl CC (2010) Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy 6:929–935. https://doi.org/10.4161/auto.6.7.12785
Kim Y, Hood DA (2017) Regulation of the autophagy system during chronic contractile activity-induced muscle adaptations. Phys Rep 5:e13307. https://doi.org/10.14814/phy2.13307
Kim Y, Memme JM, Hood DA (2018) Application of chronic stimulation to study contractile activity-induced rat skeletal muscle phenotypic adaptations. J Vis Exp 131. https://doi.org/10.3791/56827
Klionsky DJ, Abdelmohsen K, Abe A et al. (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222. https://doi.org/10.1080/15548627.2015.1100356
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z (2017) Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8:548. https://doi.org/10.1038/s41467-017-00520-9
Li H, Miao W, Ma J, Xv Z, Bo H, Li J, Zhang Y, Ji LL (2016) Acute exercise-induced mitochondrial stress triggers an inflammatory response in the myocardium via NLRP3 inflammasome activation with mitophagy. Oxidative Med Cell Longev 2016:1987149–1987111. https://doi.org/10.1155/2016/1987149
Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z (2013) Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27:4184–4193. https://doi.org/10.1096/fj.13-228486
Ljubicic V, Hood DA (2009) Specific attenuation of protein kinase phosphorylation in muscle with a high mitochondrial content. Am J Physiol Endocrinol Metab 297:E749–E758. https://doi.org/10.1152/ajpendo.00130.2009
Mansueto G, Armani A, Viscomi C, D'Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, Saha PK, Zong H, Blaauw B, Solagna F, Tezze C, Grumati P, Bonaldo P, Pessin JE, Zeviani M, Sandri M, Ballabio A (2016) Transcription factor EB controls metabolic flexibility during exercise. Cell Metab 25:182–196. https://doi.org/10.1016/j.cmet.2016.11.003
Memme JM, Oliveira AN, Hood DA (2016) Chronology of UPR activation in skeletal muscle adaptations to chronic contractile activity. Am J Phys Cell Physiol 310:C1024–C1036. https://doi.org/10.1152/ajpcell.00009.2016
Menzies KJ, Singh K, Saleem A, Hood DA (2013) Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem 288:6968–6979. https://doi.org/10.1074/jbc.M112.431155
Parousis A, Carter HN, Tran C, Erlich AT, Mesbah Moosavi ZS, Pauly M, Hood DA (2018) Contractile activity attenuates autophagy suppression and reverses mitochondrial defects in skeletal muscle cells. Autophagy 14:1886–1897. https://doi.org/10.1080/15548627.2018.1491488
Rodnick KJ, Henriksen EJ, James DE, Holloszy JO (1992) Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Phys 262:C9–C14. https://doi.org/10.1152/ajpcell.1992.262.1.C9
Saleem A, Iqbal S, Zhang Y, Hood DA (2015) Effect of p53 on mitochondrial morphology, import, and assembly in skeletal muscle. Am J Phys Cell Physiol 308:C319–C329. https://doi.org/10.1152/ajpcell.00253.2014
Scheibye-Knudsen M, Quistorff B (2009) Regulation of mitochondrial respiration by inorganic phosphate; comparing permeabilized muscle fibers and isolated mitochondria prepared from type-1 and type-2 rat skeletal muscle. Eur J Appl Physiol 105:279–287. https://doi.org/10.1007/s00421-008-0901-9
Tam BT, Pei XM, Yu AP, Sin TK, Leung KK, Au KK, Chong JT, Yung BY, Yip SP, Chan LW, Wong CS, Siu PM (2015) Autophagic adaptation is associated with exercise-induced fibre-type shifting in skeletal muscle. Acta Physiol (Oxford, England) 214:221–236. https://doi.org/10.1111/apha.12503
Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA (2015) PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle 5:9. https://doi.org/10.1186/s13395-015-0033-y
Vainshtein A, Tryon LD, Pauly M, Hood DA (2015) Role of PGC-1alpha during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Phys Cell Physiol 308:C710–C719. https://doi.org/10.1152/ajpcell.00380.2014
Yan Z, Lira VA, Greene NP (2012) Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40:159–164. https://doi.org/10.1097/JES.0b013e3182575599
Acknowledgements
We thank Jenny Lai for her dedicated technical assistance on this project.
Funding
This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) to D. A. Hood. D. A. Hood is also the holder of a Canada Research Chair in Cell Physiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were approved and performed in accordance with the guidelines of the York University Animal Care Committee.
Additional information
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Kim, Y., Triolo, M., Erlich, A.T. et al. Regulation of autophagic and mitophagic flux during chronic contractile activity-induced muscle adaptations. Pflugers Arch - Eur J Physiol 471, 431–440 (2019). https://doi.org/10.1007/s00424-018-2225-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2225-x