Abstract
Establishment of the immunological synapse (IS) between T lymphocytes and antigen-presenting cells is a key step in the adaptive immune response. Several proteins accumulate in the IS, such as the Kv1.3 potassium channel; however, the mechanism of this translocation is unknown. PSD-95 and SAP97 are adaptor proteins that regulate the polarized cell surface expression and localization of Kv1 channels in neurons. We investigated whether these proteins affect the redistribution of Kv1.3 into the IS in non-excitable human T cells. We show here that PSD-95 and SAP97 are expressed in Jurkat and interact with the C terminus of Kv1.3. Disruption of the interaction between PSD-95 or SAP97 and Kv1.3 in Jurkat was realized by the expression of a C-terminal truncated Kv1.3, which lacks the binding domain for these proteins, or by the knockdown of the expression of PSD-95 or SAP97 using specific shRNA. Expression of the truncated Kv1.3 or knockdown of PSD-95, but not the knockdown of SAP97, inhibited the recruitment of Kv1.3 into the IS; the fraction of cells showing polarized Kv1.3 expression upon engagement in an IS was significantly lower than in control cells expressing the full-length Kv1.3, and the rearrangement of Kv1.3 did not show time dependence. In contrast, Jurkat cells expressing the full-length channel showed marked time dependence in the recruitment into the IS peaking at 1 min after the conjugation of the cells. These results demonstrate that PSD-95 participates in the targeting of Kv1.3 into the IS, implying its important role in human T-cell activation.






Similar content being viewed by others
References
Affaticati P, Mignen O, Jambou F, Potier MC, Klingel-Schmitt I, Degrouard J, Peineau S, Gouadon E, Collingridge GL, Liblau R, Capiod T, Cohen-Kaminsky S (2011) Sustained calcium signalling and caspase-3 activation involve NMDA receptors in thymocytes in contact with dendritic cells. Cell Death Differ 18(1):99–108
Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, LeeHealey CJ, Andrews BS, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG (2006) Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A 103(46):17414–17419
Bock J, Szabo I, Gamper N, Adams C, Gulbins E (2003) Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem Biophys Res Commun 305(4):890–897
Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML (2001) The immunological synapse. Annu Rev Immunol 19:375–396
Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES (1999) Distinct structural requirements for clustering and immobilization of K + channels by PSD-95. J Gen Physiol 113(1):71–80
Cahalan MD, Chandy KG (2009) The functional network of ion channels in T lymphocytes. Immunol Rev 231(1):59–87
Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD (2004) K + channels as targets for specific immunomodulation. Trends Pharmacol Sci 25(5):280–289
Crabtree GR (1999) Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96(5):611–614
Doczi MA, Damon DH, Morielli AD (2011) A C-terminal PDZ binding domain modulates the function and localization of Kv1.3 channels. Exp Cell Res 317(16):2333–2341
Fanger CM, Rauer H, Neben AL, Miller MJ, Wulff H, Rosa JC, Ganellin CR, Chandy KG, Cahalan MD (2001) Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem 276(15):12249–12256
Gong J, Xu J, Bezanilla M, van Huizen R, Derin R, Li M (1999) Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science 285(5433):1565–1569
Gulbins E, Szabo I, Baltzer K, Lang F (1997) Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Natl Acad Sci U S A 94(14):7661–7666
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100
Hanada T, Lin L, Chandy KG, Oh SS, Chishti AH (1997) Human homologue of the Drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and Shaker type Kv1.3 potassium channel in T lymphocytes. J Biol Chem 272(43):26899–26904
Janeway CJ, Travers P, Walport M, Shlomchik M (2004) Immunobiology: the immune system in health and disease, 6th edn. Garland Science, New York
Jugloff DG, Khanna R, Schlichter LC, Jones OT (2000) Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem 275(2):1357–1364
Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M (1995) Clustering of Shaker-type K + channels by interaction with a family of membrane-associated guanylate kinases. Nature 378(6552):85–88
Kim E, Sheng M (1996) Differential K + channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases. Neuropharmacology 35(7):993–1000
Kuras Z, Kucher V, Gordon SM, Neumeier L, Chimote AA, Filipovich AH, Conforti L (2012) Modulation of Kv1.3 channels by protein kinase A I in T lymphocytes is mediated by the disc large 1-tyrosine kinase Lck complex. Am J Physiol Cell Physiol 302(10):C1504–1512
Leonard RJ, Garcia ML, Slaughter RS, Reuben JP (1992) Selective blockers of voltage-gated K + channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci U S A 89(21):10094–10098
Lin CS, Boltz RC, Blake JT, Nguyen M, Talento A, Fischer PA, Springer MS, Sigal NH, Slaughter RS, Garcia ML et al (1993) Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med 177(3):637–645
Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD (2008) Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A 105(6):2011–2016
Magidovich E, Orr I, Fass D, Abdu U, Yifrach O (2007) Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K + channel modulates its interaction with scaffold proteins. Proc Natl Acad Sci U S A 104(32):13022–13027
Marks DR, Fadool DA (2007) Post-synaptic density perturbs insulin-induced Kv1.3 channel modulation via a clustering mechanism involving the SH3 domain. J Neurochem 103(4):1608–1627
Martin GV, Yun Y, Conforti L (2012) Modulation of T cell activation by localized K(+) accumulation at the immunological synapse—a mathematical model. J Theor Biol 300:173–182. doi:10.1016/j.jtbi.2012.01.018
Matteson DR, Deutsch C (1984) K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature 307(5950):468–471
McCormack T, McCormack K, Nadal MS, Vieira E, Ozaita A, Rudy B (1999) The effects of Shaker beta-subunits on the human lymphocyte K + channel Kv1.3. J Biol Chem 274(29):20123–20126
Nicolaou SA, Neumeier L, Peng Y, Devor DC, Conforti L (2007) The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am J Physiol Cell Physiol 292(4):C1431–1439
Nicolaou SA, Neumeier L, Steckly A, Kucher V, Takimoto K, Conforti L (2009) Localization of Kv1.3 channels in the immunological synapse modulates the calcium response to antigen stimulation in T lymphocytes. J Immunol 183(10):6296–6302
Nicolaou SA, Neumeier L, Takimoto K, Lee SM, Duncan HJ, Kant SK, Mongey AB, Filipovich AH, Conforti L (2007) Differential calcium signaling and Kv1.3 trafficking to the immunological synapse in systemic lupus erythematosus. Cell Calcium 47(1):19–28
Nicolaou SA, Szigligeti P, Neumeier L, Lee SM, Duncan HJ, Kant SK, Mongey AB, Filipovich AH, Conforti L (2007) Altered dynamics of Kv1.3 channel compartmentalization in the immunological synapse in systemic lupus erythematosus. J Immunol 179(1):346–356
Panyi G, Deutsch C (1996) Assembly and suppression of endogenous Kv1.3 channels in human T cells. J Gen Physiol 107(3):409–420
Panyi G, Possani LD, Rodriguez de la Vega RC, Gaspar R, Varga Z (2006) K + channel blockers: novel tools to inhibit T cell activation leading to specific immunosuppression. Curr Pharm Des 12(18):2199–2220
Panyi G, Vamosi G, Bacso Z, Bagdany M, Bodnar A, Varga Z, Gaspar R, Matyus L, Damjanovich S (2004) Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci U S A 101(5):1285–1290
Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nunez L, Villalobos C, Meraner P, Becherer U, Rettig J, Niemeyer BA, Hoth M (2011) Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. Embo J 30(19):3895–3912
Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M (2007) T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A 104(36):14418–14423
Rao A, Luo C, Hogan PG (1997) Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15:707–747
Schwindling C, Quintana A, Krause E, Hoth M (2010) Mitochondria positioning controls local calcium influx in T cells. J Immunol 184(1):184–190
Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275(5296):73–77
Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS (2000) PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol 148(1):147–158
Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116(18):3475–3484
Xavier R, Seed B (2005) PDZ domains and the politics of polarity in lymphocytes. Immunity 22(6):655–656
Zhu J, Gomez B, Watanabe I, Thornhill WB (2007) Kv1 potassium channel C-terminus constant HRETE region: arginine substitution affects surface protein level and conductance level of subfamily members differentially. Mol Membr Biol 24(3):194–205. doi:10.1080/09687860601066309
Acknowledgments
The authors are thankful to Prof. Hannes Stockinger (University of Vienna, Vienna, Austria) for help in the optimization of retroviral transduction of Jurkat cells and to Prof. Jim Trimmer (University of California, Davis, USA) for the PSD-95 and SAP97 plasmids. The technical assistance of Cecilia Nagy is greatly appreciated. This project was funded by the following grants: The Hungarian Social Renewal Operational Program (TAMOP-4.2.2-A-11/1/KONV-2012-0025; TÁMOP-4.2.2/B-10/1-2010-0024; TÁMOP-4.2.2-08/1/2008-0019; TÁMOP-4.2.1/B-09/1/KONV-2010-007) and the Hungarian Scientific Research Fund (Grants K75904, CK 78179, and NK 101337). P.H. is supported by Janos Bolyai and Lajos Szodoray Fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
shRNA knockdown of PSD-95 and SAP97. Jurkat and Jurkat-mGFP-Kv1.3-WT cells were transduced with two specific shRNA constructs each to down-regulate the expression level of PSD-95 (clone 27 and 28) or SAP97 (clone 01 and 02). Scrambled shRNA was used as transduction control. In case of PSD-95 construct 27 has been chosen for further experiments. Western blot of Jurkat cell lysates using a) anti-PSD-95 (top set: WB PSD-95): 1) Jurkat, 2) Jurkat-PSD-95 knockdown clone 27 (KD-PSD-95-27), 3) Jurkat PSD-95 knockdown clone 28 (KD-PSD-95-28), 4) Jurkat scrambled shRNA control (SCR-control), 5) Jurkat stable transfected with mGFP-Kv1.3-WT, 6) Jurkat stable transfected with mGFP-Kv1.3-WT and PSD-95 knocked down, clone 27 (Jurkat- mGFP-Kv1.3-WT -KD-PSD-95-27), 7) Jurkat stable transfected with mGFP-Kv1.3-WT and PSD-95 knocked down, clone 28 (Jurkat-mGFP-Kv1.3-WT-KD-PSD-95-28), 8) Jurkat stable transfected with mGFP-Kv1.3-WT, scrambled shRNA control (Jurkat-mGFP-Kv1.3-WT-SCR-control), b) anti-SAP97 (top set: WB SAP97): 1) Jurkat, 2) Jurkat-SAP97 knockdown clone 01 (KD-SAP97-01), 3) Jurkat SAP97 knockdown clone 02 (KD-SAP97-02), 4) Jurkat scrambled shRNA control (SCR-control), 5) Jurkat stable transfected with mGFP-Kv1.3-WT, 6) Jurkat stable transfected with mGFP-Kv1.3-WT and SAP97 knocked down, clone 01 (Jurkat- mGFP-Kv1.3-WT -KD-SAP97-01), 7) Jurkat stable transfected with mGFP-Kv1.3-WT and SAP97 knocked down, clone 02 (Jurkat-mGFP-Kv1.3-WT-KD-SAP97-02), 8) Jurkat stable transfected with mGFP-Kv1.3-WT, scrambled shRNA control (Jurkat-mGFP-Kv1.3-WT-SCR-control). The actin blot (bottom set: WB actin) shows the protein quantity loaded of each total cell lysate for both figure parts (a-b). (PDF 69 kb)
Supplementary Fig. 2
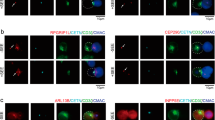
Recruitment of Kv1.3 and CD3 into the immune synapse in SAP97 knocked down or scramble control Jurkat cells. Representative examples of immune synapse formation between SEE-pulsed Raji and a) SAP97 knocked-down Jurkat expressing mGFP-tagged Kv1.3 (Jurkat-mGFP-Kv1.3-WT-KD-SAP97) or b) Jurkat transduced with scramble control shRNA. The 1st column shows the Kv1.3 signal, mGFP-tagged channels are shown in green. Jurkat cells were also labeled with anti-CD3 primary and Alexa Fluor 647 GAMIG secondary antibodies to identify the synapses (2nd column, magenta). The 3rd column shows the overlay images of the Kv1.3 and anti-CD3 fluorescence signal whereas phase contrast images of the cells forming the IS are displayed in the 4th column. (For details concerning synapse formation and fluorescent labeling see Materials and Methods.) Scale bar is 10 μm. (PDF 111 kb)
Supplementary Fig. 3
Accumulation of Kv1.3 channels in the IS is not influenced by SAP97 or non-specific shRNA knockdown. The percentage of cells showing polarized Kv1.3 expression during IS formation for Jurkat expressing the wild type (Jurkat-mGFP-Kv1.3-WT, empty bars), SAP97 knocked-down (Jurkat-mGFP-Kv1.3-WT-KD-SAP-97, for 1 min: n = 33, for 5 min: n = 41) or scramble shRNA transfected Jurkat cells (Jurkat-mGFP-Kv1.3-SCR, for 1 min: n = 29, for 5 min: n = 85) transduced the same, mGPF-tagged Kv1.3 construct. (PDF 325 kb)
Rights and permissions
About this article
Cite this article
Szilágyi, O., Boratkó, A., Panyi, G. et al. The role of PSD-95 in the rearrangement of Kv1.3 channels to the immunological synapse. Pflugers Arch - Eur J Physiol 465, 1341–1353 (2013). https://doi.org/10.1007/s00424-013-1256-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1256-6