Abstract
Purpose
To seek the threshold value of Bruch’s membrane opening-minimum rim width (BMO-MRW) where visual field (VF) damage occurs in open-angle glaucoma (OAG) and explore whether there are structural differences between primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG).
Methods
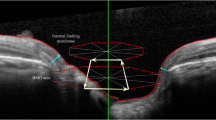
We recruited 83 healthy and 106 glaucoma (49 with POAG and 57 with NTG) subjects for this study. All subjects underwent optical coherence tomography (OCT), BMO-MRW scans, and Humphrey visual field (VF) analyzer examination. Global and sectoral BMO-MRW was correlated with the corresponding VF according to the Garway-Heath map. Using a broken-stick statistical model, the structure–function relationship of VF values and BMO-MRW, the tipping point where VF defects were associated with a reduction in BMO-MRW and the slopes above and below the tipping point were determined and compared between POAG and NTG.
Results
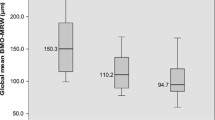
The tipping point of global BMO-MRW for VF impairment was 234.38 μm, 228.09 μm, and 249.68 μm in the OAG, POAG, and NTG groups, respectively. The slope below the tipping point was significantly steeper than the slope above it in all quadrants of each group (p < 0.001). The tipping point in NTG in the inferotemporal and nasal quadrants was smaller than that of POAG, especially in the inferotemporal quadrant.
Conclusion
In OAG, BMO-MRW loss seems to occur before the onset of perimetric impairment. Compared with POAG, NTG appears to have more severe rim damage, especially in the inferotemporal quadrant at the onset of detectable VF defects.




Similar content being viewed by others
References
Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR (2016) Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol 100(1):86–93. https://doi.org/10.1136/bjophthalmol-2015-307223
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S (2017) Glaucoma. Lancet (London, England) 390(10108):2183–2193. https://doi.org/10.1016/s0140-6736(17)31469-1
Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS (2000) Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 41(3):741–748
Quigley HA, Dunkelberger GR, Green WR (1989) Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 107(5):453–464
Ajtony C, Balla Z, Somoskeoy S, Kovacs B (2007) Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci 48(1):258–263. https://doi.org/10.1167/iovs.06-0410
Alasil T, Wang K, Yu F, Field MG, Lee H, Baniasadi N, de Boer JF, Coleman AL, Chen TC (2014) Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol 157(5):953–959. https://doi.org/10.1016/j.ajo.2014.01.014
Wollstein G, Kagemann L, Bilonick RA, Ishikawa H, Folio LS, Gabriele ML, Ungar AK, Duker JS, Fujimoto JG, Schuman JS (2012) Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol 96(1):47–52. https://doi.org/10.1136/bjo.2010.196907
Adlina AR, Alisa-Victoria K, Shatriah I, Liza-Sharmini AT, Ahmad MS (2014) Optic disc topography in Malay patients with normal-tension glaucoma and primary open-angle glaucoma. Clinical ophthalmology (Auckland, NZ) 8:2533–2539. https://doi.org/10.2147/opth.S71136
Baniasadi N, Paschalis EI, Haghzadeh M, Ojha P, Elze T, Mahd M, Chen TC (2016) Patterns of retinal nerve fiber layer loss in different subtypes of open angle glaucoma using spectral domain optical coherence tomography. J Glaucoma 25(10):865–872. https://doi.org/10.1097/ijg.0000000000000534
Kiriyama N, Ando A, Fukui C, Nambu H, Nishikawa M, Terauchi H, Kuwahara A, Matsumura M (2003) A comparison of optic disc topographic parameters in patients with primary open angle glaucoma, normal tension glaucoma, and ocular hypertension. Graefe’s Archive for Clinical and Experimental Ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 241(7):541–545. https://doi.org/10.1007/s00417-003-0702-0
Hantzschel J, Terai N, Furashova O, Pillunat K, Pillunat LE (2014) Comparison of normal- and high-tension glaucoma: nerve fiber layer and optic nerve head damage. Ophthalmol J Int d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde 231(3):160–165. https://doi.org/10.1159/000355326
Killer HE, Pircher A (2018) Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (London, England) 32(5):924–930. https://doi.org/10.1038/s41433-018-0042-2
Iester M, Mikelberg FS (1999) Optic nerve head morphologic characteristics in high-tension and normal-tension glaucoma. Arch Ophthalmol (Chicago, Ill : 1960) 117(8):1010–1013
Yamagami J, Araie M, Shirato S (1992) A comparative study of optic nerve head in low- and high-tension glaucomas. Graefe's arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 230(5):446–450
Hantzschel J, Terai N, Sorgenfrei F, Haustein M, Pillunat K, Pillunat LE (2013) Morphological and functional differences between normal-tension and high-tension glaucoma. Acta Ophthalmol 91(5):e386–e391. https://doi.org/10.1111/aos.12061
Thonginnetra O, Greenstein VC, Chu D, Liebmann JM, Ritch R, Hood DC (2010) Normal versus high tension glaucoma: a comparison of functional and structural defects. J Glaucoma 19(3):151–157. https://doi.org/10.1097/IJG.0b013e318193c45c
Hsu CH, Chen RI, Lin SC (2015) Myopia and glaucoma: sorting out the difference. Curr Opin Ophthalmol 26(2):90–95. https://doi.org/10.1097/icu.0000000000000124
Shoji T, Nagaoka Y, Sato H, Chihara E (2012) Impact of high myopia on the performance of SD-OCT parameters to detect glaucoma. Graefe's Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 250(12):1843–1849. https://doi.org/10.1007/s00417-012-1994-8
Reis AS, O’Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, Chauhan BC (2012) Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci 53(4):1852–1860. https://doi.org/10.1167/iovs.11-9309
Danthurebandara VM, Sharpe GP, Hutchison DM, Denniss J, Nicolela MT, McKendrick AM, Turpin A, Chauhan BC (2014) Enhanced structure-function relationship in glaucoma with an anatomically and geometrically accurate neuroretinal rim measurement. Invest Ophthalmol Vis Sci 56(1):98–105. https://doi.org/10.1167/iovs.14-15375
Enders P, Schaub F, Adler W, Nikoluk R, Hermann MM, Heindl LM (2017) The use of Bruch’s membrane opening-based optical coherence tomography of the optic nerve head for glaucoma detection in microdiscs. Br J Ophthalmol 101(4):530–535. https://doi.org/10.1136/bjophthalmol-2016-308957
Malik R, Belliveau AC, Sharpe GP, Shuba LM, Chauhan BC, Nicolela MT (2016) Diagnostic accuracy of optical coherence tomography and scanning laser tomography for identifying glaucoma in myopic eyes. Ophthalmology 123(6):1181–1189. https://doi.org/10.1016/j.ophtha.2016.01.052
Chauhan BC, O'Leary N, AlMobarak FA, Reis ASC, Yang H, Sharpe GP, Hutchison DM, Nicolela MT, Burgoyne CF (2013) Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology 120(3):535–543. https://doi.org/10.1016/j.ophtha.2012.09.055
Enders P, Schaub F, Adler W, Hermann MM, Dietlein TS, Cursiefen C, Heindl LM (2018) Bruch’s membrane opening-based optical coherence tomography of the optic nerve head: a useful diagnostic tool to detect glaucoma in macrodiscs. Eye (London, England) 32(2):314–323. https://doi.org/10.1038/eye.2017.306
Toshev AP, Lamparter J, Pfeiffer N, Hoffmann EM (2017) Bruch’s membrane opening-minimum rim width assessment with spectral-domain optical coherence tomography performs better than confocal scanning laser ophthalmoscopy in discriminating early glaucoma patients from control subjects. J Glaucoma 26(1):27–33. https://doi.org/10.1097/ijg.0000000000000532
Pollet-Villard F, Chiquet C, Romanet JP, Noel C, Aptel F (2014) Structure-function relationships with spectral-domain optical coherence tomography retinal nerve fiber layer and optic nerve head measurements. Invest Ophthalmol Vis Sci 55(5):2953–2962. https://doi.org/10.1167/iovs.13-13482
Reznicek L, Burzer S, Laubichler A, Nasseri A, Lohmann CP, Feucht N, Ulbig M, Maier M (2017) Structure-function relationship comparison between retinal nerve fibre layer and Bruch’s membrane opening-minimum rim width in glaucoma. Int J Ophthalmol 10(10):1534–1538. https://doi.org/10.18240/ijo.2017.10.09
Park KH, Lee JW, Kim JM, Nouri-Mahdavi K, Caprioli J (2018) Bruch’s membrane opening-minimum rim width and visual field loss in glaucoma: a broken stick analysis. Int J Ophthalmol 11(5):828–834. https://doi.org/10.18240/ijo.2018.05.19
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA (2000) Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 107(10):1809–1815
Sawada Y, Araie M, Shibata H, Ishikawa M, Iwata T, Yoshitomi T (2018) Optic disc margin anatomic features in myopic eyes with glaucoma with spectral-domain OCT. Ophthalmology 125(12):1886–1897. https://doi.org/10.1016/j.ophtha.2018.07.004
Sommer A, Miller NR, Pollack I, Maumenee AE, George T (1977) The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol (Chicago, Ill : 1960) 95(12):2149–2156
Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL 3rd, Carter-Dawson L (2002) Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res 21(1):91–125
Hood DC, Kardon RH (2007) A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 26(6):688–710. https://doi.org/10.1016/j.preteyeres.2007.08.001
Gardiner SK, Johnson CA, Demirel S (2012) The effect of test variability on the structure-function relationship in early glaucoma. Graefe's Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 250(12):1851–1861. https://doi.org/10.1007/s00417-012-2005-9
Nakatsue T, Shirakashi M, Yaoeda K, Funaki S, Funaki H, Fukushima A, Ofuchi N, Abe H (2004) Optic disc topography as measured by confocal scanning laser ophthalmoscopy and visual field loss in Japanese patients with primary open-angle or normal-tension glaucoma. J Glaucoma 13(4):291–298
Gramer E, Althaus G, Leydhecker W (1986) Site and depth of glaucomatous visual field defects in relation to the size of the neuroretinal edge zone of the optic disk in glaucoma without hypertension, simple glaucoma, pigmentary glaucoma. A clinical study with the Octopus perimeter 201 and the optic nerve head analyzer. Klinische Monatsblatter fur Augenheilkunde 189(3):190–198. https://doi.org/10.1055/s-2008-1050784
Caprioli J, Spaeth GL (1985) Comparison of the optic nerve head in high- and low-tension glaucoma. Arch Ophthalmol (Chicago, Ill : 1960) 103(8):1145–1149
Barbosa-Breda J, Van Keer K, Abegao-Pinto L, Nassiri V, Molenberghs G, Willekens K, Vandewalle E, Rocha-Sousa A, Stalmans I (2019) Improved discrimination between normal-tension and primary open-angle glaucoma with advanced vascular examinations-the Leuven Eye Study. Acta Ophthalmol 97(1):e50–e56. https://doi.org/10.1111/aos.13809
Acknowledgements
Special thanks to Xueying Li who assisted the statistical analysis in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) of Peking University First Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective study, informed consent was not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, R., Wang, X., Wei, Y. et al. Structure–function relationship between Bruch’s membrane opening-minimum rim width and perimetry in open-angle glaucoma subtypes. Graefes Arch Clin Exp Ophthalmol 258, 595–605 (2020). https://doi.org/10.1007/s00417-019-04557-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04557-y