Abstract
Background
Multiple sclerosis (MS) is a chronic demyelinating neurodegenerative disorder. Elevated levels of pro-inflammatory mediators and some oxidative stress parameters can accelerate the demyelination process. We aimed to investigate the efficacy and safety of metformin as an adjuvant therapy to interferon beta 1a (IFNβ-1a) in relapsing–remitting multiple sclerosis (RRMS) patients.
Method
Eighty RRMS patients were equally divided into 2 groups: the intervention group receiving IFNβ-1a plus 2 gm of metformin once daily and the control group receiving IFNβ-1a alone. Interleukin 17 (IL17), interleukin 22 (IL22), malondialdehyde (MDA), T2 lesions in magnetic resonance imaging (MRI) and expanded disability status scale (EDSS) were assessed at the baseline and then after 6 months.
Results
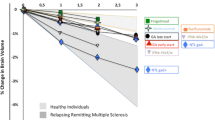
At baseline, there were no statistically significant differences between the two groups (p > 0.05). After 6 months, the change in the median (interquartile range) of the results for both the intervention and control group were; IL17 (− 1.39 (4.19) vs − 0.93 (5.48), p = 0.48), IL22 (− 0.14 (0.48) vs − 0.09 (0.6), p = 0.53), and EDSS (0 vs 0, p = 1), respectively. The mean (standard deviation) change in MDA for the intervention and control group was − 0.93 (2.2) vs − 0.5 (2.53), p = 0.038, respectively. For MRI results, 21 patients had stationary and regressive course and 1 patient had a progressive course in the intervention arm vs 12 patients had stationary and regressive course and 4 had a progressive course in the control arm, p = 0.14.
Conclusion
Adding metformin to IFNβ-1a demonstrated a potential effect on an oxidative stress marker (MDA). However, there is no statistically significant effect on immunological, MRI and clinical outcomes. We recommend larger scale studies to confirm or negate these findings.
Trial registration
ClinicalTrials.gov number: NCT05298670, 28/3/2022.




Similar content being viewed by others
Change history
18 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00415-024-12249-9
References
Chaudhuri A (2013) Multiple sclerosis is primarily a neurodegenerative disease. J Neural Transm (Vienna) 120:1463–1466. https://doi.org/10.1007/s00702-013-1080-3
Tillery EE, Clements JN, Howard Z (2017) What’s new in multiple sclerosis? Ment Health Clin 7(5):213–220
Adamczyk B, Adamczyk-Sowa M (2016) New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid Med Cell Longev. https://doi.org/10.1155/2016/1973834
Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, Ramírez-Anguiano AC, Flores-Alvarado LJ, Ramírez-Ramírez V, Macias-Islas MA, Torres-Sánchez ED (2013) Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. https://doi.org/10.1155/2013/708659
Sallusto F (2016) Heterogeneity of human CD4+ T cells against microbes. Annu Rev Immunol 34:317–334. https://doi.org/10.1146/annurev-immunol-032414-112056
Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F (2014) T helper cells plasticity in inflammation. Cytometry A 85(1):36–42. https://doi.org/10.1002/cyto.a.22348
Kolls JK, Lindén A (2004) Interleukin-17 family members and inflammation. Immunity 21(4):467–476. https://doi.org/10.1016/j.immuni.2004.08.018
Wing AC, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Sacramento PM, Andrade RM, CamargoS RF, Alves-Leon SV, Vasconcelos CC, Alvarenga R, Bento CA (2016) Interleukin-17-and interleukin-22-secreting myelin-specific CD 4+ T cells resistant to corticoids are related with active brain lesions in multiple sclerosis patients. Immunology 147(2):212–220. https://doi.org/10.1111/imm.12552
Kuntzel T, Bagnard D (2022) Manipulating Macrophage/Microglia Polarization to Treat Glioblastoma or Multiple Sclerosis. Pharmaceutics 14(2):344. https://doi.org/10.3390/pharmaceutics14020344
Mao P, Reddy PH (2010) Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta Mol Basis Dis 1802(1):66–79
Largani SHH, Borhani-Haghighi M, Pasbakhsh P, Mahabadi VP, Nekoonam S, Shiri E, Kashani IR, Zendehdel A (2019) Oligoprotective effect of metformin through the AMPK-dependent on restoration of mitochondrial hemostasis in the cuprizone-induced multiple sclerosis model. J Mol Histol 50:263–271. https://doi.org/10.1007/s10735-019-09824-0
Addabbo F, Montagnani M, Goligorsky MS (2009) Mitochondria and reactive oxygen species. Hypertension 53(6):885–892
Pardo G, Jones DE (2017) The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol 264(12):2351–2374. https://doi.org/10.1007/s00415-017-8594-9
Filipi M, Jack S (2020) Interferons in the treatment of multiple Sclerosis: A clinical efficacy, safety, and tolerability update. Int J MS Care 22(4):165–172. https://doi.org/10.7224/1537-2073.2018-063
Li H, Hu F, Zhang Y, Li K (2020) Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing–remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol 267(12):3489–3498. https://doi.org/10.1007/s00415-019-09395-w
Sattarnezhad N, Healy BC, Baharnoori M, Diaz-Cruz C, Stankiewicz J, Weiner HL, Chitnis T (2022) Comparison of dimethyl fumarate and interferon outcomes in an MS cohort. BMC Neurol 22(1):252. https://doi.org/10.1186/s12883-022-02761-8
Vermersch P, Scaramozza M, Levin S, Alroughani R, Deiva K, Pozzilli C, Lyons J, Mokliatchouk O, Pultz J, N’Dure F, Liu S, Badwan R, Branco F, Hood-Humphrey V, Franchimont N, Hanna J, Maghzi AH (2022) Effect of dimethyl fumarate vs interferon β-1a in patients with pediatric-onset multiple sclerosis: the CONNECT randomized clinical trial. JAMA Netw Open 5(9):e2230439. https://doi.org/10.1001/jamanetworkopen.2022.30439
Ning P, Luo A, Mu X, Xu Y, Li T (2022) Exploring the dual character of metformin in Alzheimer’s disease. Neuropharmacology 207:108966. https://doi.org/10.1016/j.neuropharm.2022.108966
Feng YY, Wang Z, Pang H (2023) Role of metformin in inflammation. Mol Biol Rep 50(1):789–798. https://doi.org/10.1007/s11033-022-07954-5
Buczyńska A, Sidorkiewicz I, Krętowski AJ, Zbucka-Krętowska M, Adamska A (2022) Metformin intervention—a panacea for cancer treatment? Cancers 14(5):1336
Khezri MR, Yousefi K, Mahboubi N, Hodaei D, Ghasemnejad-Berenji M (2022) Metformin in Alzheimer’s disease: An overview of potential mechanisms, preclinical and clinical findings. Biochem Pharmacol 197:114945. https://doi.org/10.1016/j.bcp.2022.114945
Tawfik TZ, Gad AH, Mehaney DA, El Nahrery EE, Shehata HS, Hashem H, Abdel Ghaffar NF, Shalaby N (2016) Interleukins 17 and 10 in a sample of Egyptian relapsing remitting multiple sclerosis patients. J Neurol Sci 369:36–38. https://doi.org/10.1016/j.jns.2016.07.034
van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, Runia TF, Jafari N, Samijn JP, de Beukelaar JWK, Wokke BHA, Siepman TAM, Hintzen RQ (2018) Application of the 2017 revised McDonald criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol 75(11): 1392-1398. 1001/jamaneurol.2018.2160
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452. https://doi.org/10.1212/wnl.33.11.1444
Cunniffe N (2021) Promoting and measuring remyelination and neuroprotection in clinical trials of people with multiple sclerosis. Dissertation, University of Cambridge. https://doi.org/10.17863/CAM.77136
Ovcharova E, Danovska M, Marinova D, Pendicheva-Duhlenska D, Tonchev P, Atanasova M, Ruseva A, Shepherd N, Tzveova R (2022) Adapted Mediterranean Diet Impact on the Symptoms of Chronic Fatigue, Serum Levels of Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Interleukin 17 (IL-17) in Patients with Relapsing-Remitting Multiple Sclerosis undergoing Disease-Modifying Therapy: A Pilot Study. J IMAB 28(1):4297–4304. https://doi.org/10.5272/jimab.2022281.4297
Noroozi S, Arababadi MK, Meimand HAE, Asadikaram G (2017) The effect of IFN-β 1a on biochemical factors in multiple sclerosis patients. Iran Red Crescent Med J 19(8):e41032
Green AE (2013) AMP-activated protein kinase (AMPK) activation for the treatment of mitochondrial disease. Dissertation, York University.
Hagen J, Zimmerman R, Goetz C, Bonnevier J, Houchins JP, Reagan K, Kalyuzhny AE (2015) Comparative multi-donor study of IFNγ secretion and expression by human PBMCs using ELISPOT side-by-side with ELISA and flow cytometry assays. Cells 4(1):84–95
Ji N, Forsthuber TG (2016) ELISPOT techniques. Methods Mol Biol 1304:63–71. https://doi.org/10.1007/7651_2014_111
Abdel-Dayem MA, Shaker ME, Gameil NM (2019) Impact of interferon β-1b, interferon β-1a and fingolimod therapies on serum interleukins-22, 32α and 34 concentrations in patients with relapsing-remitting multiple sclerosis. J Neuroimmunol 337:577062. https://doi.org/10.1016/j.jneuroim.2019.577062
Balasa R, Maier S, Voidazan S, Hutanu A, Bajko Z, Motataianu A, Tilea B, Tiu C (2017) Assessment of Interleukin-17A, Interleukin-10 and Transforming Growth Factor-Beta1 Serum Titers in Relapsing Remitting Multiple Sclerosis Patients Treated with Avonex, Possible Biomarkers for Treatment Response. CNS Neurol Disord Drug Targets 16(1):93–101. https://doi.org/10.2174/1871527315666160615110739
Toghianifar N, Ashtari F, Zarkesh-Esfahani SH, Mansourian M (2015) Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. J Neuroimmunol 285:125–128. https://doi.org/10.1016/j.jneuroim.2015.05.022
Bălaşa R, Bajko Z, Huţanu A (2013) Serum levels of IL-17A in patients with relapsing–remitting multiple sclerosis treated with interferon-β. Mult Scler 19(7):885–890. https://doi.org/10.1177/1352458512468497
Kim C, Golden SH, Mather KJ, Laughlin GA, Kong S, Nan B, Barrett-Connor E, Randolph JF (2012) Racial/ethnic differences in sex hormone levels among postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab 97(11):4051–4060
Bonnet F, Scheen A (2017) Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab 19(4):473–481. https://doi.org/10.1111/dom.12854
Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I (2013) AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol 183(2):526–541. https://doi.org/10.1016/j.ajpath.2013.04.030
Paintlia AS, Mohan S, Singh I (2013) Combinatorial effect of metformin and lovastatin impedes T-cell autoimmunity and neurodegeneration in experimental autoimmune encephalomyelitis. J Clin Cell Immunol. https://doi.org/10.4172/2155-9899.1000149
Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, Li B, Quan M, Guo L (2016) Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol 292:58–67. https://doi.org/10.1016/j.jneuroim.2016.01.014
Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182(12):8005–8014
Houshmand F, Barati M, Golab F, Ramezani-Sefidar S, Tanbakooie S, Tabatabaei M, Amiri M, Sanadgol N (2019) Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. Daru 27(2):583–592. https://doi.org/10.1007/s40199-019-00286-z
Sanadgol N, Barati M, Houshmand F, Hassani S, Clarner T, Shahlaei M, Golab F (2020) Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol Rep 72(3):641–658. https://doi.org/10.1007/s43440-019-00019-8
Abdi M, Pasbakhsh P, Shabani M, Nekoonam S, Sadeghi A, Fathi F, Abouzaripour M, Mohamed W, Zibara K, Kashani IR, Zendedel A (2021) Metformin therapy attenuates pro-inflammatory microglia by inhibiting NF-κB in cuprizone demyelinating mouse model of multiple sclerosis. Neurotox Res 39(6):1732–1746. https://doi.org/10.1007/s12640-021-00417-y
Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, Franklin RJM (2019) Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25(4):473-485e8. https://doi.org/10.1016/j.stem.2019.08.015
Negrotto L, Farez MF, Correale J (2016) Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol 73(5):520–528. https://doi.org/10.1001/jamaneurol.2015.4807
Acknowledgements
Authors would like to acknowledge the medical staff at Nasser Institute Hospital and Fatemic Cairo Hospital for their exemplary care, enthusiastic participation, and valuable support.
Author information
Authors and Affiliations
Contributions
MY-A carried out devising the main conceptual idea, study design, obtaining approval from the Scientific Research Ethics Committee at the Egyptian Ministry of Health, sample and data collection, ELISA and Spectrophotometer techniques, statistical analysis, and writing the manuscript. MH was the neurologist who provided crucial clinical data including EDSS and MRI. HM-E revised the procedures of practical techniques and commented on the manuscript. MH-S revised the study design, statistical analysis, and commented on the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The Research Ethics Committee at the Egyptian Ministry of Health approved and registered the study protocol (Com.No/Dec.No: 8-2022/5). This study was conducted according to the 1964 Declaration of Helsinki and its later amendments. All patients were educated about the study protocol and were required to sign a written informed consent before participation without any obligation to complete the study. The study was registered at “Clinical-Trials.gov” with identifier number: NCT05298670.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelgaied, M.Y., Rashad, M.H., El-Tayebi, H.M. et al. The impact of metformin use on the outcomes of relapse-remitting multiple sclerosis patients receiving interferon beta 1a: an exploratory prospective phase II open-label randomized controlled trial. J Neurol 271, 1124–1132 (2024). https://doi.org/10.1007/s00415-023-12113-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12113-2