Abstract
Background
Multiple sclerosis (MS) is an autoimmune, demyelinating disease of the central nervous system. The treatment of MS has always been a focus of neurological research. To date, the US Food and Drug Administration has approved 15 medications for modifying the course of multiple sclerosis. In this study, we examined the effects of disease-modifying therapies (DMTs) on clinical outcomes.
Methods
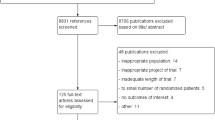
We did a systematic review and network meta-analysis based on randomized controlled trials (RCTs) comparing DMTs in patients with relapsing–remitting multiple sclerosis (RRMS). We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for RCTs published up to Oct 31, 2018. The primary outcome was efficacy (relapse rate over 24 months) and acceptability (treatment discontinuation due to adverse events over 24 months).
Findings
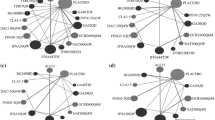
We identified 23 suitable trials encompassing 14,096 participants. During the 2 years of follow-up, all drugs were significantly more effective than were placebos. The risk ratios with 95% credible intervals were as follows: alemtuzumab, 0.49 (0.40, 0.59); ocrelizumab, 0.49 (0.40, 0.61); mitoxantrone, 0.47 (0.27, 0.80); natalizumab, 0.51 (0.43, 0.61); fingolimod, 0.57 (0.50, 0.65); peginterferon beta-1a, 0.63 (0.52, 0.77); dimethyl fumarate, 0.65 (0.56, 0.74); teriflunomide 14 mg, 0.78 (0.66, 0.92); glatiramer acetate, 0.80 (0.72, 0.89); IFN β-1a (Rebif), 0.81 (0.72, 0.90); IFN β-1b (Betaseron), 0.81 (0.72, 0.91); teriflunomide 7 mg, 0.83 (0.71, 0.98); and IFN β-1a (Avonex). 0.87 (0.77, 0.99). Risk ratios compared with placebo for discontinuation due to adverse events ranged from 1.12 for the best drug (fingolimod) to 0.10 for the worst drug (mitoxantrone); from 0.24 (alemtuzumab) to 0.89 (IFNβ-1b [Betaseron]) for sustained (3-month) disability progression; and from 0.85 (natalizumab) to 1.25 (teriflunomide 14 mg) for the number of participants with serious adverse events.
Interpretation
All DMTs were superior to placebo in reducing the relapse rate during the 2 years of follow-up. As to the comparison between drugs, alemtuzumab, ocrelizumab, natalizumab and fingolimod had a relatively higher response and lower dropout rates than did the other DMTs.


Similar content being viewed by others
References
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ (2014) Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83(11):1022–1024. https://doi.org/10.1212/WNL.0000000000000768
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391(10130):1622–1636. https://doi.org/10.1016/S0140-6736(18)30481-1
Chao MJ, Ramagopalan SV, Herrera BM, Orton SM, Handunnetthi L, Lincoln MR, Dyment DA, Sadovnick AD, Ebers GC (2011) MHC transmission: insights into gender bias in MS susceptibility. Neurology 76(3):242–246. https://doi.org/10.1212/WNL.0b013e318207b060
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378(2):169–180. https://doi.org/10.1056/NEJMra1401483
English C, Aloi JJ (2015) New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther 37(4):691–715. https://doi.org/10.1016/j.clinthera.2015.03.001
Comi G, Radaelli M, Soelberg Sørensen P (2017) Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389(10076):1347–1356. https://doi.org/10.1016/S0140-6736(16)32388-1
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24(2):96–120. https://doi.org/10.1177/1352458517751049
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 25(2):215–237. https://doi.org/10.1111/ene.13536
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree B, Gronseth GS, Haboubi M, Halper J, Hosey JP, Jones DE, Lisak R, Pelletier D, Potrebic S, Sitcov C, Sommers R, Stachowiak J, Getchius T, Merillat SA, Pringsheim T (2018) Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 90(17):777–788. https://doi.org/10.1212/WNL.0000000000005347
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree B, Gronseth GS, Haboubi M, Halper J, Hosey JP, Jones DE, Lisak R, Pelletier D, Potrebic S, Sitcov C, Sommers R, Stachowiak J, Getchius T, Merillat SA, Pringsheim T (2018) Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology 90(17):789–800. https://doi.org/10.1212/WNL.0000000000005345
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey national multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology 46(4):907–911
Mouzaki A, Tselios T, Papathanassopoulos P, Matsoukas I, Chatzantoni K (2004) Immunotherapy for multiple sclerosis: basic insights for new clinical strategies. Curr Neurovasc Res 1(4):325–340
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13(3):227–231. https://doi.org/10.1002/ana.410130302
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50(1):121–127
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58(6):840–846. https://doi.org/10.1002/ana.20703
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag B, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, Bass AD, Wynn DR, Margolin DH, Lake SL, Moran S, Palmer J, Smith MS, Compston DA (2011) Alemtuzumab versus interferon β-1a in early relapsing-remitting multiple sclerosis: post hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 10(4):338–348. https://doi.org/10.1016/S1474-4422(11)70020-5
Uitdehaag B (2018) Disability outcome measures in phase III clinical trials in multiple sclerosis. CNS Drugs 32(6):543–558. https://doi.org/10.1007/s40263-018-0530-8
Vargas DL, Tyor WR (2017) Update on disease-modifying therapies for multiple sclerosis. J Investig Med 65(5):883–891. https://doi.org/10.1136/jim-2016-000339
Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, Giovannoni G, Miller D, Rashid W, Schmierer K, Shehu A, Silber E, Young C, Zajicek J (2015) Association of British neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 15(4):273–279. https://doi.org/10.1136/practneurol-2015-001139
Berger JR, Markowitz C (2018) Deciding on the best multiple sclerosis therapy: tough choices. JAMA Neurol 75(12):1461–1462. https://doi.org/10.1001/jamaneurol.2018.2689
Tramacere I, Del Giovane C, Salanti G, D’Amico R, Filippini G (2015) Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 18(9). https://doi.org/10.1002/14651858.CD011381.pub2
Fogarty E, Schmitz S, Tubridy N, Walsh C, Barry M (2016) Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: systematic review and network meta-analysis. Mult Scler Relat Disord 9:23–30. https://doi.org/10.1016/j.msard.2016.06.001
Lucchetta RC, Tonin FS, Borba H, Leonart LP, Ferreira VL, Bonetti AF, Riveros BS, Becker J, Pontarolo R, Fernandez-Llimós F, Wiens A (2018) Disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. CNS Drugs 32(9):813–826. https://doi.org/10.1007/s40263-018-0541-5
Funding
None.
Author information
Authors and Affiliations
Contributions
HHL, FLH, YLZ and KL conceived and designed the study. HHL and FLH selected the articles and extracted the data. YLZ and KL analysed the data. HHL and FLH wrote the first draft of the manuscript. YLZ and KL interpreted the data and wrote the final version. All authors read and met the ICMJE criteria for authorship and agree with the results and conclusions of this Article.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
Not applicable. The manuscript does not contain clinical studies or patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Hu, F., Zhang, Y. et al. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing–remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol 267, 3489–3498 (2020). https://doi.org/10.1007/s00415-019-09395-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09395-w