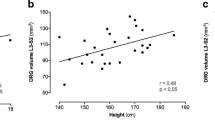
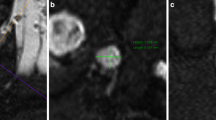
Abstract
Purpose
To examine dorsal root ganglia and the proximal nerve segments in female patients with Fabry disease by functional and morphometric magnetic resonance neurography.
Methods
In this prospective multicenter study the lumbosacral dorsal root ganglia and proximal sciatic nerve were examined in ten female patients with Fabry disease by a standardized magnetic resonance neurography protocol at 3 T. Volumes of dorsal root ganglia L3–S2, permeability of dorsal root ganglia L5 and S1 and the spinal nerve L5 as well as the cross-sectional area of the proximal sciatic nerve were compared to 16 gender-matched healthy controls.
Results
Dorsal root ganglia were symmetrically enlarged by 54% (L3), 79% (L4), 60% (L5), 94% (S1), and 106% (S2) (p < 0.001). Additionally, permeability of the blood–tissue interface was decreased by 47% (p < 0.001). This finding was most pronounced in the peripheral zone of the dorsal root ganglia, where the cell bodies of the primary sensory neurons are located (p < 0.001). While spinal nerve permeability showed no differences compared to healthy controls, proximal sciatic nerve cross-sectional area was mildly increased by 6% (p < 0.01).
Conclusion
Although heterozygous, Fabry females show severe enlarged dorsal root ganglia with a concomitant dysfunctional perfusion, even in patients with minor disease progression and in patients who are not considered for enzyme replacement therapy yet. Alterations in dorsal root ganglia volume and perfusion might serve as a very early in vivo marker for involvement of the peripheral nervous system in Fabry disease, even in patients with residual enzyme activity.



Similar content being viewed by others
References
Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunig F, Wanner C, Warnock DG, Lemay RM, Germain DP, Fabry R (2008) Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab 93(2):112–128. https://doi.org/10.1016/j.ymgme.2007.09.013
Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ (2009) Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 24(7):2102–2111. https://doi.org/10.1093/ndt/gfp031
Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, Breunig F, Charrow J, Germain DP, Nicholls K, Banikazemi M (2007) Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis 30(2):184–192. https://doi.org/10.1007/s10545-007-0521-2
Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR (2015) Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52(5):353–358. https://doi.org/10.1136/jmedgenet-2014-102797
Hughes DA, Barba Romero MA, Hollak CE, Giugliani R, Deegan PB (2011) Response of women with Fabry disease to enzyme replacement therapy: comparison with men, using data from FOS–the Fabry outcome survey. Mol Genet Metab 103(3):207–214. https://doi.org/10.1016/j.ymgme.2011.03.022
Hopkin RJ, Bissler J, Banikazemi M, Clarke L, Eng CM, Germain DP, Lemay R, Tylki-Szymanska A, Wilcox WR (2008) Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr Res 64(5):550–555. https://doi.org/10.1203/PDR.0b013e318183f132
Politei JM, Bouhassira D, Germain DP, Goizet C, Guerrero-Sola A, Hilz MJ, Hutton EJ, Karaa A, Liguori R, Uceyler N, Zeltzer LK, Burlina A (2016) Pain in Fabry disease: practical recommendations for diagnosis and treatment. CNS Neurosci Ther 22(7):568–576. https://doi.org/10.1111/cns.12542
Uceyler N, Ganendiran S, Kramer D, Sommer C (2014) Characterization of pain in fabry disease. Clin J Pain 30(10):915–920. https://doi.org/10.1097/AJP.0000000000000041
Ramaswami U, Whybra C, Parini R, Pintos-Morell G, Mehta A, Sunder-Plassmann G, Widmer U, Beck M, Investigators FOSE (2006) Clinical manifestations of fabry disease in children: data from the Fabry outcome survey. Acta Paediatr 95(1):86–92
Godel T, Baumer P, Pham M, Kohn A, Muschol N, Kronlage M, Kollmer J, Heiland S, Bendszus M, Mautner VF (2017) Human dorsal root ganglion in vivo morphometry and perfusion in Fabry painful neuropathy. Neurology 89(12):1274–1282. https://doi.org/10.1212/WNL.0000000000004396
Gadoth N, Sandbank U (1983) Involvement of dorsal root ganglia in Fabry’s disease. J Med Genet 20(4):309–312
Godel T, Pham M, Heiland S, Bendszus M, Baumer P (2016) Human dorsal-root-ganglion perfusion measured in-vivo by MRI. Neuroimage 141:81–87. https://doi.org/10.1016/j.neuroimage.2016.07.030
Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 94(12):1187–1204. https://doi.org/10.1016/j.diii.2013.10.010
Kaye EM, Kolodny EH, Logigian EL, Ullman MD (1988) Nervous system involvement in Fabry’s disease: clinicopathological and biochemical correlation. Ann Neurol 23(5):505–509. https://doi.org/10.1002/ana.410230513
Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M (2004) The Mainz Severity Score Index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 65(4):299–307. https://doi.org/10.1111/j.1399-0004.2004.00219.x
Toth C, Brussee V, Cheng C, Zochodne DW (2004) Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol 63(6):561–573
Rahman MH, Jha MK, Kim JH, Nam Y, Lee MG, Go Y, Harris RA, Park DH, Kook H, Lee IK, Suk K (2016) Pyruvate dehydrogenase kinase-mediated glycolytic metabolic shift in the dorsal root ganglion drives painful diabetic neuropathy. J Biol Chem 291(11):6011–6025. https://doi.org/10.1074/jbc.M115.699215
Choi L, Vernon J, Kopach O, Minett MS, Mills K, Clayton PT, Meert T, Wood JN (2015) The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci Lett 594:163–168. https://doi.org/10.1016/j.neulet.2015.01.084
Acknowledgements
We thank all patients for their valuable cooperation in this study. The authors thank Véronique Schwehr and Merle Brunnée for support in recruitment and examination of healthy volunteers. T.G. is supported by a postdoctoral fellowship from the Medical Faculty of the University of Heidelberg. A.K. received travel Grants and speaker honoraria from Shire, Sanofi Genzyme and Amicus. N.M. received travel Grants, research Grants and speaker honoraria from Shire, Sanofi Genzyme and Amicus. M.B. received Grants from the German Research Council (SFB 1158). J.K. received a research Grant and personal fees from Alnylam Pharmaceuticals, and personal fees from Pfizer Pharmaceuticals. S.H. was supported by a Grant from the German Research Council (SFB 1118). V.M. was supported by a Grant from the Märta and Erik Karberg Foundation for Medical Research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Godel, T., Köhn, A., Muschol, N. et al. Dorsal root ganglia in vivo morphometry and perfusion in female patients with Fabry disease. J Neurol 265, 2723–2729 (2018). https://doi.org/10.1007/s00415-018-9053-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9053-y