Abstract
Background
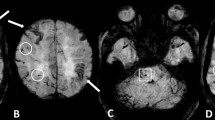
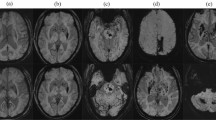
Spontaneous cerebellar-intracerebral hemorrhage (ICH) can be associated with both cerebral amyloid angiopathy (CAA) and hypertensive small vessel disease (HTN-SVD, i.e. arteriolosclerosis). To better understand the underlying microangiopathy of cerebellar-ICH, we aimed to evaluate the spatial distribution of supratentorial cerebral microbleeds (CMBs) and neuropathologic profiles in these patients.
Methods
We enrolled consecutive cerebellar-ICH patients. Clinical variables and MRI markers specific for CAA and HTN-SVD were assessed. Patients were classified into categories according to the topography (strictly-lobar, strictly-deep, and mixed) of supratentorial CMBs and comparisons were performed. Available neuropathological material was reviewed to evaluate the presence and severity of arteriolosclerosis and CAA.
Results
Ninety-eight cerebellar-ICH patients were enrolled. Fifty patients (51%) had at least one supratentorial CMB. Twelve patients (12%) had strictly lobar-CMBs, 12 patients (12%) showed strictly deep-CMBs and mixed-CMBs (lobar and deep CMBs) were present in 26 cerebellar-ICH patients (27%). In multivariable analysis, cerebellar-ICH patients with mixed-CMBs were associated with higher prevalence of hypertension (OR 4.9, 95% confidence interval [CI] 1.2–20, p = 0.017) but with lower prevalence of severe centrum-semiovale enlarged perivascular spaces (OR 0.2, CI 0.05–0.8, p = 0.024) when compared to cerebellar-ICH patients with strictly lobar-CMBs. Vascular risk factors and neuroimaging characteristics were similar between strictly deep-CMBs and mixed-CMBs. Six patients had available neuropathological material for analyses and they all showed some degree of arteriolosclerosis.
Conclusions
Cerebellar-ICH patients frequently show supratentorial CMBs. The mixed-CMBs pattern appears to be the most common. Our radiological and pathological results suggest that the majority of cerebellar-ICH patients harbor HTN-SVD as dominant microangiopathy.


Similar content being viewed by others
References
Dinsdale HB (1964) Spontaneous hemorrhage in the posterior fossa: a study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol 10:200–217
Pasi M, Marini S, Morotti A et al (2018) Cerebellar hematoma location: implications for the underlying microangiopathy. Stroke 49:207–210
Itoh Y, Yamada M, Hayakawa M et al (1993) Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 116:135–141
Pasi M, Charidimou A, Boulouis G et al (2018) Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology 90:e119–e126
Falcone GJ, Brouwers HB, Biffi A et al (2014) Warfarin and statins are associated with hematoma volume in primary infratentorial intracerebral hemorrhage. Neurocrit Care 21:192–199
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Greenberg SM, Vernooij MW, Cordonnier C et al (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8:165–174
Gregoire SM, Chaudhary UJ, Brown MM et al (2009) The Microbleed Anatomical Rating Scale (MARS) reliability of a tool to map brain microbleeds. Neurology 73:1759–1766
Caunca MR, Brutto VD, Gardener H et al (2016) cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: the Northern Manhattan Study. J Am Heart Assoc 5:e003477
Charidimou A, Boulouis G, Pasi M et al (2017) MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88:1157–1164
Charidimou A, Linn J, Vernooij MW et al (2015) Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 138:2126–2139
Auriel E, Gurol ME, Ayres A et al (2012) Characteristic distributions of intracerebral hemorrhage—associated diffusion-weighted lesions. Neurology 79:2335–2341
Charidimou A, Martinez-Ramirez S, Shoamanesh A et al (2015) Cerebral amyloid angiopathy with and without hemorrhage evidence for different disease phenotypes. Neurology 84:1206–1212
Deramecourt V, Slade JY, Oakley AE et al (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78:1043–1050
Vonsattel JPG, Myers RH, Tessa Hedley-Whyte E et al (1991) Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 30:637–649
Greenberg SM, Vonsattel J-PG (1997) Diagnosis of cerebral amyloid angiopathy: sensitivity and specificity of cortical biopsy. Stroke 28:1418–1422
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Funding
NIH Grants R01AG047975, R01AG026484, P50AG005134, K23AG02872605.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pasi, M., Charidimou, A., Boulouis, G. et al. Cerebral small vessel disease in patients with spontaneous cerebellar hemorrhage. J Neurol 266, 625–630 (2019). https://doi.org/10.1007/s00415-018-09177-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-09177-w