Abstract
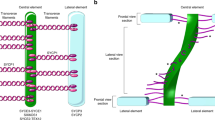
The synaptonemal complex is an evolutionarily conserved, supramolecular structure that holds the homologous chromosomes together during the pachytene stage of the first meiotic prophase. Among vertebrates, synaptonemal complex dynamics has been analyzed in mouse spermatocytes following the assembly of its components from leptotene to pachytene stages. With few exceptions, a detailed study of the disassembly of SCs and the behavior of SC components at recombination sites at the onset of diplotene has not been accomplished. Here, we describe for the first time the progressive disassembly of the SC in chicken oocytes during the initial steps of desynapsis using immunolocalization of specific SC proteins and super-resolution microscopy. We found that transverse filament protein SYCP1 and central element component SYCE3 remain associated with the lateral elements at the beginning of chromosomal axis separation. As the separation between lateral elements widens, these proteins eventually disappear, without any evidence of subsequent association. Our observations support the idea that post-translational modifications of the central region components have a role at the initial phases of the SC disassembly. At the crossover sites, signaled by persistent MLH1 foci, the central region proteins are no longer detected when the SYCP3-positive lateral elements are widely separated. These findings are indicative that SC disassembly follows a general pattern along the desynaptic bivalents. The present work shows that the use of avian oocytes at prophase I provides a valuable model to explore the time course and chromosomal localization of SC proteins and its relationship with local changes along meiotic bivalents.





Similar content being viewed by others
References
Bolcun-Filas E, Handel MA (2018) Meiosis: the chromosomal foundation of reproduction. Biol Reprod 99(1):112–126
Cahoon CK, Hawley RS (2016) Regulating the construction and demolition of the synaptonemal complex. Nat Struct Mol Biol 23(5):369–377
Costa Y, Cooke HJ (2007) Dissecting the mammalian synaptonemal complex using targeted mutations. Chromosom Res 15(5):579–589
Davies OR, Maman JD, Pellegrini L (2012) Structural analysis of the human SYCE2-TEX12 complex provides molecular insights into synaptonemal complex assembly. Open Biol 2:120099
del Priore L, Pigozzi MI (2012) Chromosomal axis formation and meiotic progression in chicken oocytes: a quantitative analysis. Cytogenet Genome Res 137:15–21
del Priore L, Pigozzi MI (2016) Meiotic recombination analysis in female ducks (Anas platyrhynchos). Genetica 144(3):307–312. https://doi.org/10.1007/s10709-016-9899-9 Erratum in: Genetica. 2016 Oct;144(5):625
Dresser ME, Giroux CN (1988) Meiotic chromosome behavior in spread preparations of yeast. J Cell Biol 106:561–513
Dunce JM, Dunne OM, Ratcliff M, Millán C, Madgwick S, Usón I, Davies OR (2018) Structural basis of meiotic chromosome synapsis through SYCP1 self-assembly. Nat Struct Mol Biol 25(7):557–569
Fraune J, Schramm S, Alsheimer M, Benavente R (2012) The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res 318:1340–1346
Fraune J, Brochier-Armanet C, Alsheimer M, Volff JN, Schücker K, Benavente R (2016) Evolutionary history of the mammalian synaptonemal complex. Chromosoma 125(3):355–356
Gaginskaya ER, Chin SH (1980) Peculiarities of oogenesis in the chicken. II Follicular period in oocyte development. Ontogenes (Russ) 11:213–221
Gao J, Colaiácovo MP (2017) Zipping and unzipping: protein modifications regulating synaptonemal complex dynamics. Trends Genet 34(3):232–245
Guioli S, Lovell-Badge R, Turner JM (2012) Error-prone ZW pairing and no evidence for meiotic sex chromosome inactivation in the chicken germ line. PLoS Genet 8:e1002560
Hamant O, Ma H, Cande WZ (2006) Genetics of meiotic prophase I in plants. Annu Rev Plant Biol 57:267–302
Hernández-Hernández A, Masich S, Fukuda T, Kouznetsova A, Sandin S, Daneholt B, Höög C (2016) The central element of the synaptonemal complex in mice is organized as a bilayered junction structure. J Cell Sci 129(11):2239–2249
Hutchison N (1987) Lampbrush chromosomes of the chicken. J Cell Biol 105:1493–1500
Jordan PW, Karppinen J, Handel MA (2012) Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes. J Cell Sci 125(Pt 21):5061–5072
Loidl J (2003) Chromosomes of the budding yeast Saccharomyces cerevisiae. Int Rev Cytol 222:141–196
Moses MJ (1956) Chromosomal structures in crayfish spermatocytes. J Biophys Biochem Cytol 2(2):215–218
Moses MJ (1968) Synaptonemal complex. Annu Rev Genet 2:363–412
Pigozzi MI (2001) Distribution of MLH1 foci on the synaptonemal complexes of chicken oocytes. Cytogenet Cell Genet 95(3–4):129–133
Pigozzi MI (2016) The chromosomes of birds during meiosis. Cytogenet Genome Res 150(2):128–138
Pigozzi MI, Solari AJ (1999a) Recombination nodule mapping and chiasma distribution in spermatocytes of the pigeon, Columba livia. Genome 42:308–314
Pigozzi MI, Solari AJ (1999b) Equal frequencies of recombination nodules in both sexes of the pigeon suggest a basic difference with eutherian mammals. Genome 42:315–321
Qiao H, Chen JK, Reynolds A, Höög C, Paddy M, Hunter N (2012) Interplay between synaptonemal complex, homologous recombination, and centromeres during mammalian meiosis. PLoS Genet 8(6):e1002790
Rahn MI, Solari AJ (1986) Recombination nodules in the oocytes of the chicken, Gallus domesticus. Cytogenet Cell Genet 43:187–193
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682
Schramm S, Fraune J, Naumann R, Hernandez-Hernandez A, Höög C, Cooke HJ, Alsheimer M, Benavente R (2011) A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination and fertility. PLoS Genet 7:e1002088
Schücker K, Holm T, Franke C, Sauer M, Benavente R (2015) Elucidation of synaptonemal complex organization by super-resolution imaging with isotropic resolution. Proc Natl Acad Sci U S A 112(7):2029–2033
Sciurano RB, Solari AJ (2014) Ultrastructural and immunofluorescent methods for the study of XY body as a biomarker. In: Stockert, Espada, Blázquez-Castro (eds) Functional analysis of DNA and chromatin, methods in molecular biology, Chapter 11, vol 2094. Springer Science + Bussiness Media, NY, pp 137–149
Solari AJ (1977) Ultrastructure of the synaptic autosomes and the ZW bivalent in chicken oocytes. Chromosoma 64:155–165
Solari AJ (1992) Equalization of z and w axes in chicken and quail oocytes. Cytogenet Cell Genet 59:52–56
Winkel K, Alsheimer M, Öllinger R, Benavente R (2009) Protein SYCP2 provides a link between transverse filaments and lateral elements of mammalian synaptonemal complexes. Chromosoma 118(2):259–267
Yang F, Wang PJ (2009) The mammalian synaptonemal complex: a scaffold and beyond. Genome Dyn 5:69–80
Zickler D, Kleckner N (2015) Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb Perspect Biol 7(6)
Acknowledgements
We would like to especially thank Prof. Dr. Alberto Solari for his continuous encouraging and helpful discussions, MSc. Mónica Rahn and MSSc C. Deparci for her helpful assistance, Dr. Teresa Klein for helpful counseling on SIM, and MSc. Irene da Cruz and Marcus Behringer for technical discussions on SIM techniques. We would also like to thank Prof. Dr. Manfred Alsheimer for the generous gift of SYCE3 antibody.
Funding
PIP1148CO from the National Council of Scientific and Technical Research (CONICET to R.B.S.-A.J.S.); Grant Be1168/8-1 from the German Science Foundation (to R.B.); BID-PICT 2016N° 2302 (ANPCyT to MIP); Research Stays for University Academics and Scientists (DAAD to RBS, 2017); International Scholarship for Young Researchers (CONICET to RBS, 2018).
Author information
Authors and Affiliations
Contributions
RBS and MIP conceived the study; RBS, performed research; RBS, MIP, and RB analyzed the data and wrote the draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a Special Issue on “Recent advances in meiosis from DNA replication to chromosome segregation” edited by Valérie Borde and Francesca Cole, co-edited by Paula Cohen and Scott Keeney".
Electronic supplementary material
Fig. S1
Measurements of the distance between axial elements in points I, II, III and IV of the macrochromosomes #3 and #8 at initial and advanced stages of chromosomes separation. Both macrochromosomes were straightened using the corresponding tool from the FIJI image software and four points (I, II, III and IV) were measured along the chromosomal axes. Those regions with twisted axes were discarded. Scale bars: 2 μm and 1 μm (insets) (PNG 1100 kb)
Rights and permissions
About this article
Cite this article
Sciurano, R.B., Pigozzi, M.I. & Benavente, R. Disassembly of the synaptonemal complex in chicken oocytes analyzed by super-resolution microscopy. Chromosoma 128, 443–451 (2019). https://doi.org/10.1007/s00412-019-00693-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-019-00693-w